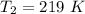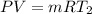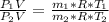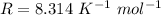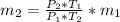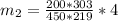Physics, 25.12.2020 17:20 claudioocampo5621
An insulated rigid tank with a volume of 0.57 m3, contains 4 kg of Argon gas at 450 kPa and 30 C. A valve is now opened, and the Argon is slowly allowed to escape until the pressure inside drops to 200 kPa. Assuming the Argon remaining inside the tank has undergone a reversible, adiabatic process, determine the final mass in the tank,

Answers: 2


Another question on Physics

Physics, 21.06.2019 23:20
In the sport of parasailing, a person is attached to a rope being pulled by a boat while hanging from a parachute-like sail. a rider is towed at a constant speed by a rope that is at an angle of 15 ∘ from horizontal. the tension in the rope is 1900 n. the force of the sail on the rider is 30∘ from horizontal. what is the weight of the rider? express your answer with the appropriate units.
Answers: 1

Physics, 22.06.2019 06:30
If an atom contains 11 protons in its nucleus, predict the element with the atomic number and electronic configuration. will the number of electrons remain the same during the chemical reaction in such an atom? explain.
Answers: 3

Physics, 22.06.2019 16:30
Acoil suspended freely, points in some direction when no current is passed through it . can you tell what will happen when a current is passed though it?
Answers: 3

Physics, 22.06.2019 22:00
It takes a lot of energy to get the temperature of water to increase and eventually boil because water has a high heat.
Answers: 1
You know the right answer?
An insulated rigid tank with a volume of 0.57 m3, contains 4 kg of Argon gas at 450 kPa and 30 C. A...
Questions

Mathematics, 10.12.2020 17:50




Advanced Placement (AP), 10.12.2020 17:50

Mathematics, 10.12.2020 17:50

Mathematics, 10.12.2020 17:50


Mathematics, 10.12.2020 17:50



Mathematics, 10.12.2020 17:50

Mathematics, 10.12.2020 17:50

Chemistry, 10.12.2020 17:50


Spanish, 10.12.2020 17:50




Mathematics, 10.12.2020 17:50

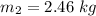
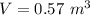
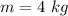
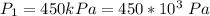
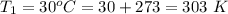
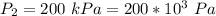
![T_2 = T_1 * [\frac{P_2}{P_1} ]^{ \frac{(k - 1 )}{k} }](/tpl/images/1007/5612/a5d79.png)
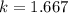
![T_2 = 303 * [\frac{200}{450} ]^{ \frac{(1.667- 1 )}{1.667} }](/tpl/images/1007/5612/8aae2.png)