
Physics, 28.12.2020 14:00 zeesharpe05
When 1.34 g Zn(s) reacts with 60.0 mL of 0.750 M HCl(aq), 3.14 kJ of heat are produced. Determine the enthalpy change per mole of zinc reacting for the reaction: Zn(s) +2HCl (aq) → ZnCl2 (aq) +H2 (g)

Answers: 1


Another question on Physics

Physics, 22.06.2019 02:50
Steam is generated in a boiler of a cogeneration plant at 10 mpa and 450°c at a steady rate of 5 kg/s. in normal operation, steam expands in a turbine to a pressure of 0.5 mpa and is then routed to the process heater, where it supplies the process heat. steam leaves the process heater as a saturated liquid and is pumped to the boiler pressure. in this mode, no steam passes through a condenser, which operates at 20 kpa. (a) determine the power produced in the turbine and the rate at which process heat is supplied in this mode. (b) determine the power produced in the turbine and the rate of process heat supplied if only 60 percent of the steam is routed to the process heater and the remainder is expanded to the condenser pressure. (3.32 mw; 9.69 mw; 4.25 mw; 5.82 mw)
Answers: 3

Physics, 22.06.2019 06:20
Three charge are arranged as shown in the diagram. the magnitude of the net electrical force acting on the +6 uc charge, rounded to the tenths place, is .
Answers: 1

Physics, 22.06.2019 08:00
If the force applied to an object remains constant, is more power needed for the object to move faster ? explain
Answers: 3

Physics, 22.06.2019 12:00
Explain why electric current cannot exist if a current doesn't have a voltage source.
Answers: 2
You know the right answer?
When 1.34 g Zn(s) reacts with 60.0 mL of 0.750 M HCl(aq), 3.14 kJ of heat are produced. Determine th...
Questions

Mathematics, 17.10.2019 21:30


Chemistry, 17.10.2019 21:30


English, 17.10.2019 21:30


Law, 17.10.2019 21:30

Biology, 17.10.2019 21:30

Health, 17.10.2019 21:30





Computers and Technology, 17.10.2019 21:30

Computers and Technology, 17.10.2019 21:30





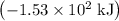 per mole
per mole 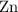 . (The negative value suggests the release of heat.) Assumption: the reaction was complete.
. (The negative value suggests the release of heat.) Assumption: the reaction was complete.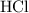 . Hence, either of the two species could be the limiting reactant. Calculating the number of moles of
. Hence, either of the two species could be the limiting reactant. Calculating the number of moles of 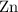 that was actually consumed requires finding the limiting reactant.
that was actually consumed requires finding the limiting reactant.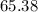 .
.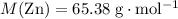 .
.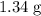 of
of 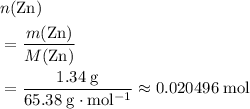 .
.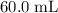 of
of 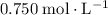 (
(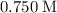 )
) 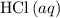 solution. Start by converting the unit of volume to liters (so as to match the unit of the concentration of this solution.)
solution. Start by converting the unit of volume to liters (so as to match the unit of the concentration of this solution.)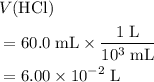 .
.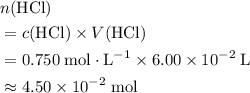 .
. .
.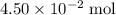 of
of 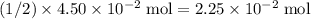
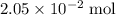 of
of 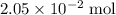 of
of 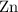 atoms would take part in this reaction.
atoms would take part in this reaction.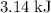 of heat through this reaction.
of heat through this reaction.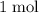 of
of 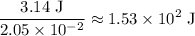 .
.

