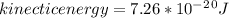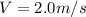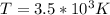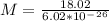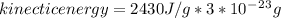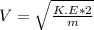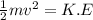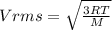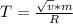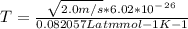The latent heat of vaporization for water at room temperature is 2430 J/g.
1. How much kinetic energy does each water molecule that evaporates possess before it evaporates?
2. Find the pre-evaporation rms speed of a water molecule that is evaporating.
3. What is the effective temperature of these molecules (modeled as if they were already in a thin gas)?
4. Why do these molecules not burn you
a. These molecules got to be slow-moving in collisions that made other molecules fast-moving; the average molecular energy decreases.
b. These molecules got to be slow-moving in collisions that made other molecules fast-moving; the average molecular energy is unaffected.
c. These molecules got to be fast-moving in collisions that made other molecules slow-moving; the average molecular energy is unaffected.
d. These molecules got to be fast-moving in collisions that made other molecules slow-moving; the average molecular energy increases.

Answers: 2


Another question on Physics

Physics, 21.06.2019 17:10
The dissociation energy of a molecule is the energy required tobreak apart the molecule into its separate atoms. the dissociationenergy for a particular molecule is 5.48 x 10-18 j.suppose that this energy is provided by a single photon. determinethe (a) wavelength and (b) frequency of the photon
Answers: 1

Physics, 21.06.2019 21:30
Ahydroelectric plant takes energy from water and turns it into electrical energy. what are the transformations of energy in the water molecules that are used in the process of generating electricity this way?
Answers: 3


Physics, 22.06.2019 16:00
The amount of potential energy possessed by an elevated object is equal to what?
Answers: 1
You know the right answer?
The latent heat of vaporization for water at room temperature is 2430 J/g.
1. How much kinetic ener...
Questions

History, 15.01.2021 17:50

Mathematics, 15.01.2021 17:50


Arts, 15.01.2021 17:50


Physics, 15.01.2021 17:50


Mathematics, 15.01.2021 17:50


Chemistry, 15.01.2021 17:50

Social Studies, 15.01.2021 17:50

Mathematics, 15.01.2021 17:50


English, 15.01.2021 17:50


Mathematics, 15.01.2021 17:50



English, 15.01.2021 17:50