
Physics, 12.01.2021 08:40 mmvill0809
A 300.0 g sample of water at 80.0°C is mixed with 300.0 g of water at
10.0°C. Assuming no heat loss to the surroundings, what is the final
temperature of the mixture? The specific heat capacity of liquid water is
4180 J/kg.°C *
Will give brainliest and lot of stars please help

Answers: 2


Another question on Physics

Physics, 22.06.2019 02:50
Steam is generated in a boiler of a cogeneration plant at 10 mpa and 450°c at a steady rate of 5 kg/s. in normal operation, steam expands in a turbine to a pressure of 0.5 mpa and is then routed to the process heater, where it supplies the process heat. steam leaves the process heater as a saturated liquid and is pumped to the boiler pressure. in this mode, no steam passes through a condenser, which operates at 20 kpa. (a) determine the power produced in the turbine and the rate at which process heat is supplied in this mode. (b) determine the power produced in the turbine and the rate of process heat supplied if only 60 percent of the steam is routed to the process heater and the remainder is expanded to the condenser pressure. (3.32 mw; 9.69 mw; 4.25 mw; 5.82 mw)
Answers: 3


Physics, 22.06.2019 14:40
How does an observation about an object differ from an inference about that object
Answers: 1

Physics, 22.06.2019 16:00
The electric field direction is defined by the direction of the force felt by (select one of the following answers): 1. a negative charge. 2. a positive charge. 3. both positive and negative charges.
Answers: 2
You know the right answer?
A 300.0 g sample of water at 80.0°C is mixed with 300.0 g of water at
10.0°C. Assuming no heat loss...
Questions

Physics, 18.11.2020 06:50








Biology, 18.11.2020 06:50

Mathematics, 18.11.2020 06:50




Computers and Technology, 18.11.2020 06:50

Health, 18.11.2020 06:50

Mathematics, 18.11.2020 06:50

History, 18.11.2020 06:50



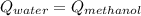
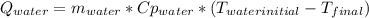
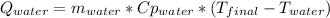
![0.3*4180*(80-T_{final})=0.3*4180*(T_{final}-10)\\100320-1254*T_{final}=1254*T_{final}-12540\\112860=2508*T_{final}\\T_{final}=45[C]](/tpl/images/1028/6130/52afa.png)


