A 0.4000 kg sample of methanol at 16.0ºC is mixed with 0.4000 kg of water at 85.0ºC. Assuming no heat loss to the surroundings, what is the final temperature of the mixture? The specific heat of methanol is 2450 J/kg•ºC, the specific heat capacity of liquid water is 4180 J/kg⋅°C

Answers: 3


Another question on Physics

Physics, 22.06.2019 12:20
Electric field of the earth. the earth has a net electric charge that causes a field at points near its surface equal to 150 n> c and directed in toward the center of the earth. (a) what magnitude and sign of charge would a 60-kg human have to acquire to overcome his or her weight by the force exerted by the earth’s electric field? (b) what would be the force of repulsion between two people each with the charge calculated in part (a) and separated by a distance of 100 m? is use of the earth’s electric field a feasible means of flight? why or why not?
Answers: 2

Physics, 22.06.2019 12:40
Question part points submissions used suppose that 2 j of work is needed to stretch a spring from its natural length of 26 cm to a length of 36 cm. (a) how much work is needed to stretch the spring from 28 cm to 32 cm? (round your answer to two decimal places.)
Answers: 2

Physics, 22.06.2019 14:00
Agraduated cylinder contains 63.0 ml of water. a piece of gold, which has a density of 19.3 g/ cm3, is added to the water and the volume goes up to 64.5 ml. calculate the mass in grams of the gold that was added to the water. explain how you got your answer.
Answers: 3

Physics, 22.06.2019 14:00
If element x has 99 protons how many electrons does it have
Answers: 1
You know the right answer?
A 0.4000 kg sample of methanol at 16.0ºC is mixed with 0.4000 kg of water at 85.0ºC. Assuming no hea...
Questions



Physics, 16.04.2020 20:55

Biology, 16.04.2020 20:55

English, 16.04.2020 20:55

Geography, 16.04.2020 20:55




History, 16.04.2020 20:55


Mathematics, 16.04.2020 20:55

Physics, 16.04.2020 20:55



Mathematics, 16.04.2020 20:55

History, 16.04.2020 20:55



Mathematics, 16.04.2020 20:55

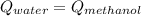
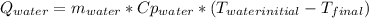
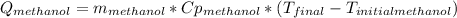
![0.4*4180*(85-T_{final})=0.4*2450*(T_{final}-16)\\142120-1672*T_{final}=980*T_{final}-15680\\157800=2652*T_{final}\\T_{final}=59.5[C]](/tpl/images/1031/5237/e4a4e.png)