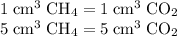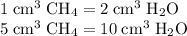Physics, 16.01.2021 18:30 schapethan
A mixture of 5 cm3 of CH4 and 100 cm3 of air is exploded. Assume air is 80% N2 by volume and 20% O2 by volume. The resulting mixture is cooled. All volumes are measured at room temperature and pressure.
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
What is the composition of the resulting gas?
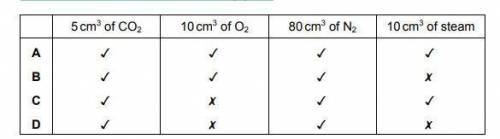

Answers: 3


Another question on Physics

Physics, 22.06.2019 03:00
An equinox occurs when a. neither end of earth's axis is tilted toward or away from the sun. b. the north end of earth's axis is tilted away from the sun. c. the north end of earth's axis is tilted toward the sun. d. earth's axis is parallel to the sun's rays. select the best answer from the choices provided a b c d
Answers: 1

Physics, 22.06.2019 10:10
Apair of 10μf capacitors in a high-power laser are charged to 1.7 kv.a. what charge is stored in each capacitor? b. how much energy is stored in each capacitor?
Answers: 2

Physics, 22.06.2019 13:00
Which of the following correctly describes what happens when an atomic bomb explodes? small pieces of fissionable material are joined and form a body with a mass greater than the critical mass, the relative number of neutrons escaping decreases, and a chain reaction and explosion result. large pieces of fissionable matter are brought together quickly and form a body with a mass smaller than the critical mass, the relative number of escaping neutrons increases, and a chain reaction and explosion result.
Answers: 2

Physics, 22.06.2019 15:00
What pressure difference is required between the ends of a 2.0-m-long, 1.0-mm-diameter horizontal tube for 40 c water to flow through it at an average speed of 4.0 m/s?
Answers: 1
You know the right answer?
A mixture of 5 cm3 of CH4 and 100 cm3 of air is exploded. Assume air is 80% N2 by volume and 20% O2...
Questions

Biology, 23.07.2019 11:30

Chemistry, 23.07.2019 11:30



Spanish, 23.07.2019 11:30




Mathematics, 23.07.2019 11:30

Chemistry, 23.07.2019 11:30

English, 23.07.2019 11:30

English, 23.07.2019 11:30

Social Studies, 23.07.2019 11:30

Spanish, 23.07.2019 11:30


Health, 23.07.2019 11:30

Health, 23.07.2019 11:30



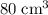 Nitrogen,
Nitrogen, 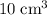 oxygen,
oxygen, 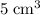 carbon dioxide, and
carbon dioxide, and 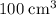 . Thus, the volume of oxygen and nitrogen in the air has been:
. Thus, the volume of oxygen and nitrogen in the air has been: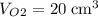
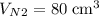
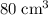 .
.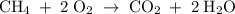
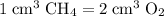
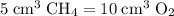
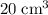 , the remaining oxygen after the reaction has been
, the remaining oxygen after the reaction has been