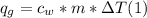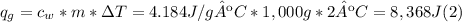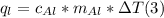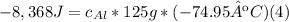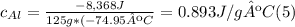Introduction: The specific heat capacity of a substance is the amount of energy needed to change the temperature of that substance by 1 °C. Specific heat capacity can be calculated using the following equation:
q = mc deltaT
In the equation q represents the amount of heat energy gained or lost in joules), m is the mass of the substance (in grams), c is the specific heat capacity of the substance (in J/g °C), and AT is the temperature change of the substance in °C).
Goal: Calculate the specific heat capacities of copper, granite, lead, and ice.
Solve: When you mix two substances, the heat gained by one substance is equal to the heat lost by the other substance. Suppose you place 125 g of aluminum in a calorimeter with 1,000 g of water. The water changes temperature by 2 °C and the aluminum changes temperature by -74.95 °C.
A. Water has a known specific heat capacity of 4.184 J/g °C. Use the specific heat equation to find out how much heat energy the water gained (q).
B. Assume that the heat energy gained by the water is equal to the heat energy lost by the aluminum. Use the specific heat equation to solve for the specific heat of aluminum. Aluminum's accepted specific heat value is 0.900 J/g °C. Use this value to check your work.

Answers: 1


Another question on Physics

Physics, 22.06.2019 03:00
An internally reversible refrigerator has a modified coefficient of performance accounting for realistic heat transfer processes of where qin is the refrigerator cooling rate, qout is the heat rejection rate, and is the power input. show that copm can be expressed in terms of the reservoir temperatures tc and th, the cold and hot thermal resistances rt,c and rt,h, and qin, as where rtot rt,c rt,h. also, show that the power input may be expressed as 1.39 a household refrigerator operates with cold- and hot-temperature reservoirs of tc 5 c and th 25 c, respectively. when new, the cold and hot side resistances are rc,n 0.05 k/w and rh,n 0.04 k/w, respectively. over time, dust accumulates on the refrigerator’s condenser coil, which is located behind the refrigerator, increasing the hot side resistance to rh,d 0.1 k/w. it is desired to have a refrigerator cooling rate of qin 750 w. using the results of problem 1.38, determine the modified coefficient of performance and the required power input w under (a) clean and (b) dusty coil conditions. internally reversible refrigerator qout qin w high-temperature reservoir low-temperature reservoir th th,i tc,i tc high-temperature side resistance low-temperature side resistance w qin th tc qinrtot tc qinrtot copm tc qinrtot th tc
Answers: 2

Physics, 22.06.2019 10:20
Electromagnetic induction. a coil of wire contains n turns and has an electrical resistance r. the radius of each turn is a. initially, inside the coil there exists a uniform magnetic field of magnitude b0 parallel to the axis of the coil. the magnetic field is then reduced slowly. the current induced in the coil is i. how long does it take for the magnitude of the uniform field to drop to zero?
Answers: 1

Physics, 22.06.2019 17:30
Which of the choices are parts of an atom? select all that apply. a.) compound b.) electron c.) neutron d.) molecule e.) photon f.) proton g.) element
Answers: 1

Physics, 22.06.2019 18:00
Astudent pushes a 60-n block across the floor for a distance of 10 m. how much work was done to move the block
Answers: 1
You know the right answer?
Introduction: The specific heat capacity of a substance is the amount of energy needed to change the...
Questions

History, 20.09.2019 23:30

Mathematics, 20.09.2019 23:30



Health, 20.09.2019 23:30

Biology, 20.09.2019 23:30

Social Studies, 20.09.2019 23:30


Arts, 20.09.2019 23:30


Health, 20.09.2019 23:30


Social Studies, 20.09.2019 23:30

Mathematics, 20.09.2019 23:30




Chemistry, 20.09.2019 23:30

Mathematics, 20.09.2019 23:30