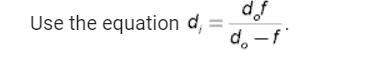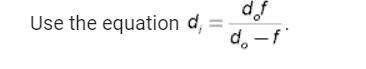Physics, 19.01.2021 14:00 billythe1st
Magnesium is a shiny, flexible metal that can be burned in the presence of air.
When it is burned, it produces a white powder that is no longer shiny or flexible.
This white powder also has more mass than the original magnesium. *
The molecules were separated into individual atoms.
The mass of the magnesium changed.
Smoke that was trapped between the magnesium atoms was given off.
A new substance wa formed
Explain why you chose the above answer. *
Your answer

Answers: 1


Another question on Physics

Physics, 22.06.2019 05:30
James is trying to prove newton's 2nd law of motion. he tries to move four different objects with different masses, shapes, and sizes from point a to point b. the objects are a toy car, a car, a refrigerator, and a kitchen table. after some experimentation, he finds that force is dependent on the mass of the object, rather than it's size or shape. the object that takes the most force to move is the
Answers: 1

Physics, 22.06.2019 10:00
Which atomic model was proposed as a result of j. j. thomson’s work?
Answers: 1

Physics, 22.06.2019 14:30
Aracecar driver has to hold on tightly when going around a banked curve. approximately what is the centripetal force on a 2220.0 kg car going around a circle with a diameter of 190.0 meters at 25.0 m/s?
Answers: 1

Physics, 22.06.2019 23:30
An astronaut on the moon throws a baseball upward. the astronaut is 6 ft, 6 in. tall, and the initial velocity of the ball is 3030 ft per sec. the height s of the ball in feet is given by the equation s equals negative 2.7 t squared plus 30 t plus 6.5s=−2.7t2+30t+6.5, where t is the number of seconds after the ball was thrown. complete parts a and b.
Answers: 2
You know the right answer?
Magnesium is a shiny, flexible metal that can be burned in the presence of air.
When it is burned,...
Questions

Mathematics, 11.11.2021 03:30


Law, 11.11.2021 03:30

English, 11.11.2021 03:30


Mathematics, 11.11.2021 03:30




Mathematics, 11.11.2021 03:30



Chemistry, 11.11.2021 03:30

English, 11.11.2021 03:30

Social Studies, 11.11.2021 03:30

Chemistry, 11.11.2021 03:30


Arts, 11.11.2021 03:30


SAT, 11.11.2021 03:30