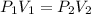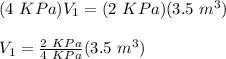Julie blows a bubble. At first, the pressure of the gas in the bubble is 4kPa. The bubble floats into the air and expands. When it gets to the top of a tree the bubble has a pressure of 2kPa and a volume of 3.5m³. Assuming a constant temperature, what was the volume in m³ of the bubble when it was first blown?

Answers: 1


Another question on Physics

Physics, 21.06.2019 17:10
An air-standard stirling cycle operates with a maximum pressure of 600 psia and a minimum pressure of 10 psia. the maximum volume of the air is 10 times the minimum volume. the temperature during the heat rejection process is 100°f. calculate the specific heat added to and rejected by this cycle, as well as the net specific work produced by the cycle. use constant specific heats at room temperature. the properties of air at room temperature are r
Answers: 2

Physics, 22.06.2019 01:30
Ajet plane has a sound level of 120 db at a distance of 60 m. what is the q23. sound level at a distance of 6.0 km? (60)2 (6000)? om solution 80db db, hint: 16x assume ultrasound waves travel through the body of an animal at 1540 q24. m/s. if a 30,000 hz signal were reflected off a portion of a heart which was moving toward the source at 3 m/s, what frequency signal would return to the stationary source? solution 30.117hz
Answers: 3

Physics, 22.06.2019 08:00
Ms.hidalgo opens the door to her classroom. her classroom is 60 degrees and the air outside is 80. predict what will happen using your knowledge of how heat flows.
Answers: 2

Physics, 22.06.2019 14:00
Explain why you think this diagram shows what happened to the carbon in the biodome.
Answers: 2
You know the right answer?
Julie blows a bubble. At first, the pressure of the gas in the bubble is 4kPa. The bubble floats int...
Questions

Mathematics, 02.10.2019 09:00

Mathematics, 02.10.2019 09:00



Chemistry, 02.10.2019 09:00


Health, 02.10.2019 09:00

English, 02.10.2019 09:00

English, 02.10.2019 09:00

History, 02.10.2019 09:00

Mathematics, 02.10.2019 09:00

Spanish, 02.10.2019 09:00

Mathematics, 02.10.2019 09:00

Mathematics, 02.10.2019 09:00

Mathematics, 02.10.2019 09:00




Biology, 02.10.2019 09:00