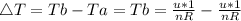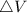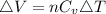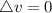Consider a system consisting of an ideal gas confined within a container, one wall of which is a movable piston. Energy can be added to the gas in the form of heat by applying a flame to the outside of the container. Conversely, energy can also be removed from the gas in the form of heat by immersing the container in ice water. Energy can be added to the system in the form of work by pushing the piston in, thereby compressing the gas. Conversely, if the gas pushes the piston out, thereby pushing some atmosphere aside, the internal energy of the gas is reduced by the amount of work done.

Answers: 3


Another question on Physics

Physics, 21.06.2019 20:30
Air enters a compressor operating at steady state at 1.05 bar, 300 k, with a volumetric flow rate of 93 m3/min and exits at 12 bar, 400 k. heat transfer occurs at a rate of 15.5 kw from the compressor to its surroundings. assuming the ideal gas model for air neglecting kinetic potential energy effects, determine the power in put in kw.
Answers: 2

Physics, 22.06.2019 14:40
During the experiment if you could triple the breakaway magnetic force with all other quantities left unchanged, what is the new value for the critical velocity if it was v0 (initial velocity), initially? (b) now if you halved the radius with all other quantities left unchanged, what is the new critical velocity if it was v0 (initial velocity), initially? (c) if during the experiment, critical velocity quadrupled with all other quantities left unchanged, what is the new breakaway force if its magnitude was initially f0,?
Answers: 1


Physics, 22.06.2019 22:20
The starship enterprise is caught in a time warp and spock is forced to use the primitive techniques of the 20th century to determine the specific heat capacity of an unknown mineral. the 125-g sample was heated to 96.3°c and placed into a calorimeter containing 85.9 g of water at 20.0°c. the heat capacity of the calorimeter was 14.2 j/k. the final temperature in the calorimeter was 24.5°c. what is the specific heat capacity (in j/g°c) of the mineral? enter to 4 decimal places.
Answers: 1
You know the right answer?
Consider a system consisting of an ideal gas confined within a container, one wall of which is a mov...
Questions




Mathematics, 28.03.2021 17:50

English, 28.03.2021 17:50





History, 28.03.2021 17:50

Mathematics, 28.03.2021 17:50

Mathematics, 28.03.2021 17:50

English, 28.03.2021 17:50

English, 28.03.2021 17:50

Geography, 28.03.2021 17:50

Mathematics, 28.03.2021 17:50

Computers and Technology, 28.03.2021 17:50



Mathematics, 28.03.2021 17:50

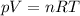
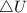 as the system of ideal gas goes from point A to point B on the graph recall u is proportional to T
as the system of ideal gas goes from point A to point B on the graph recall u is proportional to T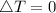
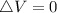
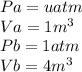
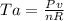
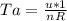
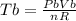
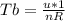
 is mathematically given as
is mathematically given as