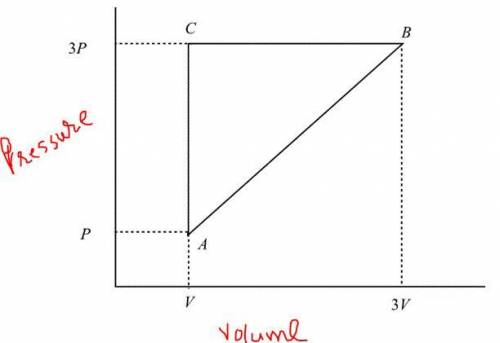
A cylinder with initial volume V contains a sample of gas at pressure p. On one end of the cylinder, a piston is let free to move so that the gas slowly expands in such a way that its pressure is directly proportional to its volume. After the gas reaches the volume 3V and pressure 3p, the piston is pushed in so that the gas is compressed isobarically to its original volume V. The gas is then cooled isochorically until it returns to the original volume and pressure. Find the work W done on the gas during the entire process. Find the amount of work W done on the gas during the entire process. Express your answer in terms of some or all of the variables p and V.

Answers: 2


Another question on Physics

Physics, 22.06.2019 11:10
An isotope undergoes radioactive decay by emitting radiation that has a –1 charge. what other characteristic does the radiation have?
Answers: 3

Physics, 22.06.2019 12:30
Write a full page that sumerizes thermodynamics it’s from the website visionlearnig
Answers: 1

Physics, 22.06.2019 15:30
Two pans of a balance are 24.1 cm apart. the fulcrum of the balance has been shifted 1.33 cm away from the center by a dishonest shopkeeper. by what percentage is the true weight of the goods being marked up by the shopkeeper? assume the balance has negligible mass. answer in units of %.
Answers: 1

Physics, 22.06.2019 17:00
If a negatively charged particle is placed at rest in an electric potential field that increases in the positive x-direction, what will the particle do? a. accelerate in the positive x-direction b. remain at rest c. accelerate in the negative x-direction
Answers: 3
You know the right answer?
A cylinder with initial volume V contains a sample of gas at pressure p. On one end of the cylinder,...
Questions


English, 26.04.2021 21:00


Mathematics, 26.04.2021 21:00


Mathematics, 26.04.2021 21:00

English, 26.04.2021 21:00

Social Studies, 26.04.2021 21:00


Physics, 26.04.2021 21:00

Biology, 26.04.2021 21:00




History, 26.04.2021 21:00



Mathematics, 26.04.2021 21:00

Biology, 26.04.2021 21:00

History, 26.04.2021 21:00