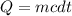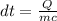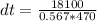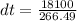Physics, 16.02.2021 02:40 NightSun8891
A 567-g empty iron kettle is put in a hot stove the kettle absorbs 18,100 j of heat to raise its temperature from 15.0c to a final temperature what is the final temperature? The specific heat capacity of iron is 470j/kg•k

Answers: 3


Another question on Physics


Physics, 22.06.2019 09:30
Which are advantages of renewable resources? check all that apply. renewable energy supplies are completely reliable everywhere. some renewable resources will never be used up. little or no waste is produced by renewable resource plants. electricity can be generated in large quantities. many renewable energy facilities have lower operating costs.
Answers: 1

Physics, 22.06.2019 14:00
How much energy must a refrigerator absorb from 225 g of water so that the temperature of the water will drop from 35°c to 5°c
Answers: 3

Physics, 22.06.2019 15:50
An object with initial temperature 130 ∘ f is submerged in large tank of water whose temperature is 50 ∘ f . find a formula for f ( t ) , the temperature of the object after t minutes, if the cooling constant is k = − 0.2 . remember newton's law of cooling (the rate of change of temperature with respect to time is equal to k times the difference between the temperature of the object and the surrounding temperature) ! : )
Answers: 1
You know the right answer?
A 567-g empty iron kettle is put in a hot stove the kettle absorbs 18,100 j of heat to raise its tem...
Questions

Mathematics, 23.10.2021 02:50



Computers and Technology, 23.10.2021 02:50



Geography, 23.10.2021 02:50



Business, 23.10.2021 02:50

Health, 23.10.2021 02:50


Mathematics, 23.10.2021 02:50



Mathematics, 23.10.2021 02:50



English, 23.10.2021 02:50