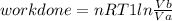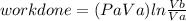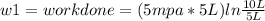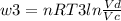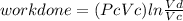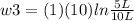Physics, 19.02.2021 17:00 bkimswift7
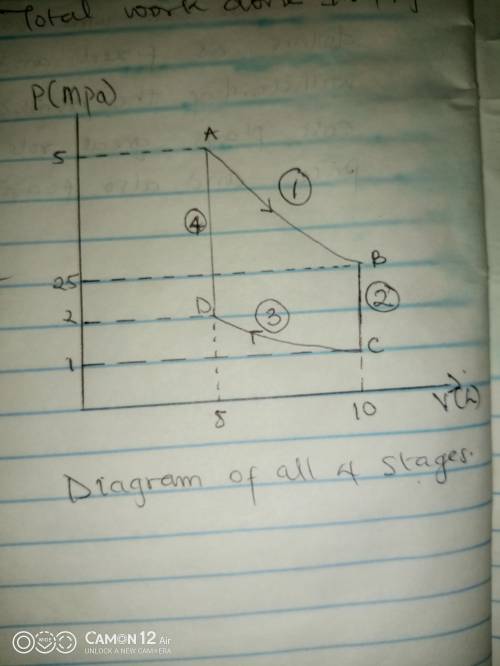
Thermodynamic Processes Two moles of a monatomic ideal gas at (5 MPa, 5 L) is expanded isothermally until the volume is doubled (step 1). Then it is cooled isochorically until the pressure is 1 MPa (step 2). The temperature drops in this process. The gas is now compressed isothermally until its volume is back to 5 L, but its pressure is now 2 MPa (step 3). Finally, the gas is heated isochorically to return to the initial state (step 4). (a) Draw the four processes in the pV plane. (b) Find the total work done by the gas.

Answers: 1


Another question on Physics

Physics, 22.06.2019 05:30
What do you think car designers do if the damage caused by a crash test is too severe?
Answers: 1

Physics, 22.06.2019 08:30
Brutus, the dog, is pulling a bone to the left with a force of 20 n. lassie, another dog, is pulling a bone to the right with a force of 18n. what is the net force? a. b. c.
Answers: 1

Physics, 22.06.2019 14:00
Often called simply "velocity," this is the velocity of an object at a particular moment in time.
Answers: 1

You know the right answer?
Thermodynamic Processes
Two moles of a monatomic ideal gas at (5 MPa, 5 L) is expanded isothermally...
Questions

Computers and Technology, 21.02.2020 02:16









Computers and Technology, 21.02.2020 02:17

Mathematics, 21.02.2020 02:17

History, 21.02.2020 02:17





Computers and Technology, 21.02.2020 02:18

Mathematics, 21.02.2020 02:18