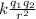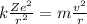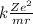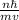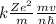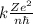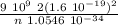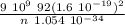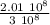Physics, 22.02.2021 23:20 Lovergirl13
Express the speed of the electron in the Bohr model in terms of the fundamental constants (me, e, h, e0), the nuclear charge Z, and the quantum number n. Evaluate the speed of an electron in the ground states of He1 ion and U911. Compare these speeds with the speed of light c. As the speed of an object approaches the speed of light, relativistic effects become important. In which kinds of atoms do you expect relativistic effects to be greatest

Answers: 2


Another question on Physics

Physics, 22.06.2019 08:30
An automobile steering wheel is shown. what is the ideal mechanical advantage? if the ama is 8, what is the efficiency of the steering wheel?
Answers: 1

Physics, 22.06.2019 09:00
A100 kg running back runs at 5m/s into a stationary linebacker. it takes 0.5 for the running back to be completely stopped
Answers: 3

Physics, 22.06.2019 23:30
1. explain how you calculate the net force in any direction on the box. 2. suppose an upward force of 15n is added to the box. what will be the net vertical force on the box? 3. what force could be applied to the box to make the net force in the horizontal direction zero? explain. 4. suppose a force of 25n to the right is added to the box. what will be the net force to the right?
Answers: 3

You know the right answer?
Express the speed of the electron in the Bohr model in terms of the fundamental constants (me, e, h,...
Questions




History, 09.10.2019 09:00


Chemistry, 09.10.2019 09:00

History, 09.10.2019 09:00



Chemistry, 09.10.2019 09:00



History, 09.10.2019 09:00



Mathematics, 09.10.2019 09:00

Mathematics, 09.10.2019 09:00