
Physics, 24.02.2021 18:10 shelbybibb99
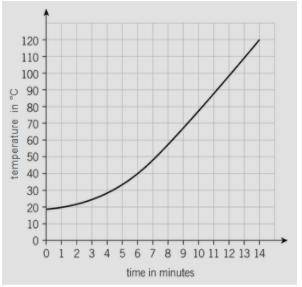
The mass of the block is 1kg. Calculate the specific heat capacity of the metal when 3.9 × 10^4 joules of energy are transferred to the metal block between 6 and 14 minutes. (Give your answer as a number only)

Answers: 2


Another question on Physics

Physics, 21.06.2019 21:30
Aquantity of gas has a volume of 1.5 m3 and an absolute pressure of 95 kpa. when the gas is compressed to a volume of 0.5 m3, what is the new absolute pressure of the gas? (assume that there’s no change in temperature.)
Answers: 3

Physics, 22.06.2019 12:50
Air is contained in a variable-load piston-cylinder device equipped with a paddle wheel. initially, air is at 400 kpa and 17°c. the paddle wheel is now turned by an external electric motor until 75 kj/kg of work has been transferred to air. during this process, heat is transferred to maintain a constant air temperature while allowing the gas volume to triple. calculate the required amount of heat transfer in kj/kg.
Answers: 2

Physics, 22.06.2019 19:30
Water is siphoned from a large tank and discharges into the atmosphere through a 50-mm diameter tube. the end of the tube is b = 2.6 m below the tank bottom which is a = 6.7 m deep, and viscous effects are negligible. determine the maximum height h over which the water can be siphoned without cavitation occurring. atmospheric pressure is 101.4 kpa, and the water vapor pressure is 1.79 kpa (absolute). report your answer in meters to two decimal places.
Answers: 1

Physics, 22.06.2019 19:30
Coal contains energy. a. light b. kinetic c. chemical d. mechanical
Answers: 1
You know the right answer?
The mass of the block is 1kg. Calculate the specific heat capacity of the metal when 3.9 × 10^4 joul...
Questions


Mathematics, 03.02.2020 00:55

Mathematics, 03.02.2020 00:55

Mathematics, 03.02.2020 00:55


Physics, 03.02.2020 00:55

History, 03.02.2020 00:55

Biology, 03.02.2020 00:55



History, 03.02.2020 00:55

Mathematics, 03.02.2020 00:55


Mathematics, 03.02.2020 00:55

History, 03.02.2020 00:55





Mathematics, 03.02.2020 00:55



