Answers: 3


Another question on Physics

Physics, 22.06.2019 05:30
Aforklift raises a 1,020 n crate 3.50 m up to a shelf. how much work is done by the forklift on the crate? the forklift does j of work on the crate.
Answers: 2

Physics, 22.06.2019 17:30
Four objects each with charge +2.0×10−7c are located at the corners of a square whose sides are 2.0 m long. part a what quantities can be determined using this information? check all that apply. the electric force on a charged object placed at the center of the square. the mass of each object. the total electric potential energy of the system consisting of the four charged objects. part b find the electric force on a charged object placed at the center of the square.
Answers: 1

Physics, 22.06.2019 18:30
Which form of cell division creates the sperm and egg, resulting in half of the chromosomes as the other cells?
Answers: 1

Physics, 22.06.2019 21:40
Ahair dryer is basically a duct in which a few layers of electric resistors are placed. a small fan pulls the air in and forces it through the resistors where it is heated. air enters a 1200 w hair dryer at 100 kpa and 22°c and leaves at 47°c. the cross-sectional area of the hair dryer at the exit is 60 cm2. neglecting the power consumed by the fan and the heat losses through the walls of the hair dryer, determine (a) the volume flow rate of air at the inlet and (b) the velocity of the air at the exit.
Answers: 1
You know the right answer?
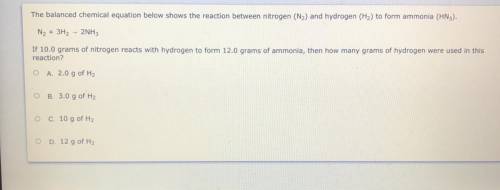
PLZZ HURRY WILL MARK BRIANLIST N2 + 3H2 + 2NH3
If 10.0 grams of nitrogen reacts with hydrogen to fo...
Questions




Physics, 05.03.2022 22:00

Chemistry, 05.03.2022 22:00

English, 05.03.2022 22:00

History, 05.03.2022 22:00




Mathematics, 05.03.2022 22:10


Chemistry, 05.03.2022 22:10



Physics, 05.03.2022 22:10


Mathematics, 05.03.2022 22:10


English, 05.03.2022 22:20