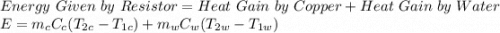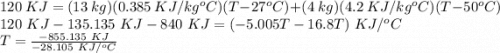Physics, 12.03.2021 15:20 dolphinkina35
A system consists of a copper tank whose mass is 13 kg, 4 kg of liquid water, and an electrical resistor of negligible mass. The system is insulated on its outer surface. Initially, the temperature of the copper is 27oC and the temperature of the water is 50oC. The electrical resistor transfers 120 kJ of energy to the system. Eventually the system comes to equilibrium. Determine the final equilibrium temperature (in oC).

Answers: 2


Another question on Physics

Physics, 21.06.2019 23:50
Select the correct answer from each drop-down menu. compared to its surroundings, the concentration of solutes is low inside a cell. so, the cell is with respect to its surroundings. a particular solute in this cell uses energy for its transport from the cell to its surroundings. this type of transport is called
Answers: 3


Physics, 22.06.2019 09:00
When a light bulb shines, it gives off light energy and energy. a. heat b. potential c. chemical d. electrical
Answers: 2

Physics, 22.06.2019 15:30
At 20∘c, the hole in an aluminum ring is 2.800 cm in diameter. you need to slip this ring over a steel shaft that has a room-temperature diameter of 2.804 cm . 1. to what common temperature should the ring and the shaft be heated so that the ring will just fit onto the shaft? coefficients of linear thermal expansion of steel and aluminum are 12×10−6 k−1 and23×10−6 k−1 respectively.
Answers: 1
You know the right answer?
A system consists of a copper tank whose mass is 13 kg, 4 kg of liquid water, and an electrical resi...
Questions



Mathematics, 17.09.2019 20:00


History, 17.09.2019 20:00



Mathematics, 17.09.2019 20:00


English, 17.09.2019 20:00



Mathematics, 17.09.2019 20:00


Mathematics, 17.09.2019 20:00

English, 17.09.2019 20:00

Mathematics, 17.09.2019 20:00


History, 17.09.2019 20:00