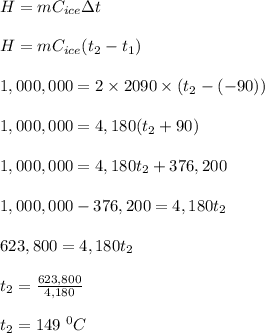The specific heat of water in its solid phase (ice) is 2090 J/(kg K), while in the liquid phase (water) its specific heat is 4190 J/(kg K). Water's latent heat of fusion is 333,000 J/kg. If you have a 2kg block of ice at -90°C and you add 1,000,000 J of heat, what is its new temperature?
a. 0°C
b. 14°C
c. 49°C
d. 149°C

Answers: 1


Another question on Physics

Physics, 21.06.2019 17:00
Which energy transformation occurs in the core of a nuclear reactor? nuclear energy mechanical energy nuclear energy - thermal energy thermal energy -- nuclear energy mechanical energy - nuclear energy
Answers: 2

Physics, 22.06.2019 05:00
Asmall 21 kilogram canoe is floating downriver at a speed of 1 m/s. what is the canoe's kinetic energy?
Answers: 1

Physics, 22.06.2019 14:10
What is the weight of a 8-kg substance in n, kn, kg·m/s2, kgf, lbm·ft/s2, and lbf?
Answers: 2

Physics, 22.06.2019 17:00
Simon is writing a story about an astronaut whose spacecraft has been boarded by space pirates. the astronaut has her lucky penny in her hand behind her back as the space pirates break into the control room. she has just locked the controls so that the ship is accelerating in the direction of the control room’s ceiling. simon wants the astronaut to use the penny to hit a button on the control panel to turn off the lights and escape. the button is located a short distance behind and below the astronaut’s hands. how should simon use the theory of relativity to describe what the astronaut must do in order to hit the button?
Answers: 1
You know the right answer?
The specific heat of water in its solid phase (ice) is 2090 J/(kg K), while in the liquid phase (wat...
Questions



Mathematics, 25.03.2021 01:00



Biology, 25.03.2021 01:00


Mathematics, 25.03.2021 01:00





Computers and Technology, 25.03.2021 01:00

History, 25.03.2021 01:00

English, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00

Business, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00