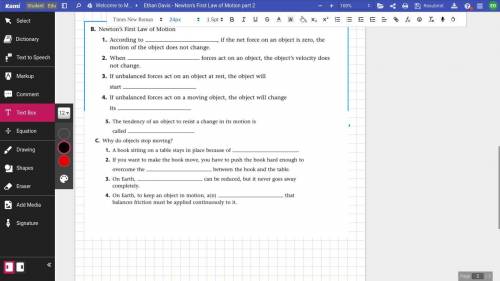
Answer as many as you can please up to 4 if no more then I shall report the (London accent)
...

Physics, 02.04.2021 07:00 vanessa051266
Answer as many as you can please up to 4 if no more then I shall report the (London accent)

Answers: 1


Another question on Physics

Physics, 22.06.2019 10:00
Because air contracts as it cools, the air pressure inside a freezer is typically lower than on the outside. why do ice cubes inside a freezer tend to shrink over time? a. the ice dissolves oxygen from the air, forming a denser crystalline matrix.b. the ice reacts chemically with carbon dioxide in the air, forming gaseous carbon compounds.c. the ice melts, and then the liquid freezes as ice crystals on the bottom of the freezer.d. the ice sublimes, and then the water vapor deposits as ice crystals on the sides of the freezer.
Answers: 1

Physics, 22.06.2019 14:00
Why is rain likely when warm, moisture-laden air meets cold air? a) the lighter warm air will rise and cool down, causing condensation and rain. b) the cold air moves faster and pushes the warm air away, causing condensation and rain. c) the moisture in the warm air condenses on contact with the cold air, causing rain to fall. d) the cold air mixes with the warm air, reducing its temperature causing moisture to condense.
Answers: 1

Physics, 22.06.2019 17:40
Emmy kicks a soccer ball up at an angle of 45° over a level field. she watches the ball's trajectory and notices that it lands, two seconds after being kicked, about 20 m away to the north. assume that air resistance is negligible, and plot the horizontal and vertical components of the ball's velocity as a function of time. consider only the time that the ball is in the air, after being kicked but before landing. take "north" and "up" as the positive ‑ and ‑directions, respectively, and use ≈10 m/s2 for the downward acceleration due to gravity.
Answers: 2

Physics, 22.06.2019 18:50
An insulated thermos contains 148 g of water at 72.7 ˚c. you put in a 11.7 g ice cube at 0.00 ˚c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg•k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?
Answers: 2
You know the right answer?
Questions

Health, 17.09.2019 17:30


Chemistry, 17.09.2019 17:30


Computers and Technology, 17.09.2019 17:30


History, 17.09.2019 17:30

Mathematics, 17.09.2019 17:30


English, 17.09.2019 17:30


English, 17.09.2019 17:30


Mathematics, 17.09.2019 17:30

Mathematics, 17.09.2019 17:30



Mathematics, 17.09.2019 17:30

Mathematics, 17.09.2019 17:30

Mathematics, 17.09.2019 17:30



