
Physics, 04.02.2020 18:46 hihihi129473838
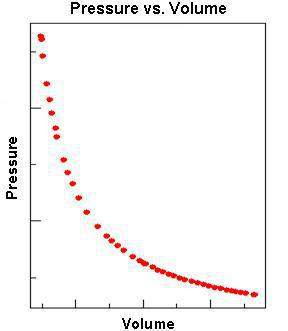
The scatter plot above shows the relationship between the pressure and volume of a gas (at fixed temperature). which of the following conclusions can be drawn?
a. the pressure of a gas decreases as its volume decreases.
b. there is no relationship between the pressure and volume of a gas.
c. the pressure of a gas increases as its volume decreases.
d. as the volume of a gas increases, the pressure of the gas stays constant.

Answers: 2


Another question on Physics

Physics, 22.06.2019 02:00
What is created when solids,liquids,an gases mix with one another
Answers: 1

Physics, 22.06.2019 15:20
Aphoton is absorbed by an electron that is in the n = 3 state of a hydrogen atom, causing the hydrogen atom to become ionized. very far away from the nucleus, the released electron has a velocity of 750,000 m/s. what was the wavelength of the absorbed photon?
Answers: 2

Physics, 23.06.2019 00:30
Why does the equilibrium position of the spring change when a mass is added to the spring? will the mass oscillate around the new equilibrium position of the spring or the previous position without a mass attached to the spring? if the equilibrium position of the spring changes by 20 cm (assuming no initial mass) when a mass is added to the spring with constant 4.9 kg/s^2, what is the mass of the object attached to the spring?
Answers: 1

Physics, 23.06.2019 07:00
The doctor lands his tardis on a planet whose radius is 1.2x10^7m the acceleration due to gravity is 18m/s^2. what is the mass of the planet
Answers: 1
You know the right answer?
The scatter plot above shows the relationship between the pressure and volume of a gas (at fixed tem...
Questions

Mathematics, 23.11.2021 14:00

Business, 23.11.2021 14:00

Mathematics, 23.11.2021 14:00



English, 23.11.2021 14:00

History, 23.11.2021 14:00

Chemistry, 23.11.2021 14:00

Biology, 23.11.2021 14:00








Computers and Technology, 23.11.2021 14:00


Spanish, 23.11.2021 14:00

Mathematics, 23.11.2021 14:00



