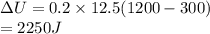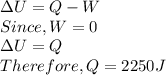A closed, rigid container holding 0.2 moles of a monatomic ideal gas is placed over a Bunsen burner and heated slowly, starting at a temperature of 300 K. The initial pressure of the ideal gas is atmospheric pressure, and the final pressure is four times the initial pressure.
Determine the following:
a. the change in the internal energy of the gas.
b. the work done by the gas.
c. the heat flow into or out of the gas.

Answers: 2


Another question on Physics

Physics, 22.06.2019 04:10
Unpolarized light of intensity so passes through two sheets of polarizing material whose transmission axes make an angle of 60∘ with each other as shown in the figure. what is the intensity of the transmitted beam, st?
Answers: 3

Physics, 22.06.2019 12:00
Under the action of a constant force an object accelerates at 7.8 m/s2. what will the acceleration be if (a) the force is halved? (b) the object's mass is halved? (c) the force and the object's mass are both halved? (d) the force is halved and the object's mass is doubled?
Answers: 3

Physics, 22.06.2019 18:00
Sunidhi made a study chart about changes in states of matter. which headings best complete the chart?
Answers: 1

Physics, 22.06.2019 21:10
The junction rule describes the conservation of which quantity? note that this rule applies only to circuits that are in a steady state.
Answers: 1
You know the right answer?
A closed, rigid container holding 0.2 moles of a monatomic ideal gas is placed over a Bunsen burner...
Questions



Mathematics, 05.02.2021 01:10

Mathematics, 05.02.2021 01:10




Spanish, 05.02.2021 01:10

Mathematics, 05.02.2021 01:10


Physics, 05.02.2021 01:10

Chemistry, 05.02.2021 01:10






Physics, 05.02.2021 01:10


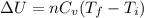
![\frac{P_1V_1}{P_2V_2} =\frac{T_i}{T_f}\\Since, V_1=V_2 [isochoric/process]\\\Rightarrow \frac{P_{atm}}{4P_{atm}} = \frac{300}{T_f} \\\Rightarrow T_f = 1200 K](/tpl/images/1264/7369/67375.png)