
Physics, 02.10.2019 17:40 zhenhe3423
The pressure exerted on a sample of a fixed amount of gas is doubled at constant temperature, and then the temperature of the gas in kelvins is doubled at constant pressure.
what is the final volume of the gas?
what is the final volume of the gas?
the final volume is twice the initial volume.
the final volume of the gas is one-fourth the initial volume.
the final volume of the gas is the same as the initial volume.
the final volume of the gas is four times the initial volume.
the final volume of the gas is one-half the initial volume.

Answers: 1


Another question on Physics

Physics, 21.06.2019 19:30
Note: the following questions are unrelated to the balmer series or the martian. refer to your course notes. a sun-sized star will spend most of its lifetime as a: white dwarf b. red giant c. protostar d. main-sequence star
Answers: 2

Physics, 22.06.2019 06:30
Air initially at 0.75 bar, 1000 k, and occupying a volume of 0.12 m^3 undergoes two processes. process 1-2: the air is compressed isothermally until the volume is halved. process 2-3: the air undergoes a constant pressure process until the volume is halved again. assume ideal gas behavior. a) determine the mass of the air, in kg. b) the work and the heat transfer for each of the two processes, in kj. (100 kj = 1 bar . m^3)
Answers: 1

Physics, 22.06.2019 07:00
Abucket full of water weights 4 kg and water well is 10m deep. a girl draws water from the well. it takes the girl 3 minutes to draw a bucket full of water from the well. what is the power of the girl?
Answers: 1

Physics, 22.06.2019 10:30
Astone weighing 1.5 kilograms is resting on a rock at a height of 20 meters above the ground. the stone rolls down 10 meters and comes to rest on a patch of moss. the gravitational potential energy of the stone on the moss is joules.
Answers: 1
You know the right answer?
The pressure exerted on a sample of a fixed amount of gas is doubled at constant temperature, and th...
Questions

Chemistry, 07.07.2019 16:00


Mathematics, 07.07.2019 16:00

English, 07.07.2019 16:00


English, 07.07.2019 16:00

History, 07.07.2019 16:00


Mathematics, 07.07.2019 16:00

Mathematics, 07.07.2019 16:00


Business, 07.07.2019 16:00




Mathematics, 07.07.2019 16:00

Business, 07.07.2019 16:00



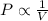 (at constant temperature and number of moles)
(at constant temperature and number of moles)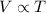 (at constant pressure and number of moles)
(at constant pressure and number of moles)

