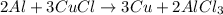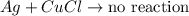Physics, 18.01.2020 18:31 isabelacarraler
In an experiment the chemical reaction between a piece of aluminum foil and copper(ii)chloride solution in a beaker is observed. the aluminum seems to disapear over time. the balanced chemical equation is given by: 2 al + 3 cucl ⇒3 cu +2 alcl3
can you predict the outcome of the same experiment when aluminum is replaced with a more reactive element such as silver?
a) no reaction would occur.
b) the silver would disappear but no solid precipitate would form.
c) the silver would disappear and a silver solid precipitate would form.
d) the silver would disappear and a brownish red solid precipitate would form.
hint: what would the new product be?

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:20
Which quantities are scalars? choose all that apply a.distance b.speed c. acceleration d.velocity
Answers: 2

Physics, 23.06.2019 02:40
Estimate the kinetic energy of the neptune with respect to the sun as the sum of the terms, that due to its daily rotation about its axis, and that due to its yearly revolution about the sun. [assume the neptune is a uniform sphere with mass = 1.0×1026 kg , radius = 2.5×1010 m , rotation period 16.1 h , orbital period 6.01×104 d and is 4.6×109 km from the sun.]
Answers: 2

Physics, 23.06.2019 08:00
(10 pts! ) the major factor which causes seasonal weather changes on the earth is: a. elliptical orbit b. variation in distance from sun c. tilt of the axis d. atmosphere
Answers: 2

You know the right answer?
In an experiment the chemical reaction between a piece of aluminum foil and copper(ii)chloride solut...
Questions

Arts, 10.03.2021 19:40

Geography, 10.03.2021 19:40

Mathematics, 10.03.2021 19:40

Mathematics, 10.03.2021 19:40

Social Studies, 10.03.2021 19:40

Physics, 10.03.2021 19:40



Mathematics, 10.03.2021 19:40




Social Studies, 10.03.2021 19:40

Mathematics, 10.03.2021 19:40

Mathematics, 10.03.2021 19:40

Spanish, 10.03.2021 19:40

Mathematics, 10.03.2021 19:40


Mathematics, 10.03.2021 19:40