The equation
p1v1/t1 = p2v2/t2 is often used to study the volume of a gas at different pressu...

The equation
p1v1/t1 = p2v2/t2 is often used to study the volume of a gas at different pressures and temperatures. a scientist studying a gas found that p1v1/t1 = 44 atmospheres*liters/kelvin. if the values for standard temperature and pressure are used for the variables p1 and t1, what is the value of v1?
a) 12 liters
b) 129 liters
c) 1,200 liters
d) 12,019 liters

Answers: 1


Another question on Physics

Physics, 22.06.2019 12:00
If two students are running down the hall toward each other, trying to get to class, and they have the same mass and acceleration, what will happen when they collide? will their forces cancel out or will each one experience a reaction?
Answers: 1

Physics, 22.06.2019 14:00
Estimate the change in the gibbs energy and molar gibbs energy of 1.0dm3 of octane when the pressure acting on it is increased from 1.0 atm to 100 atm. the mass density of octane is 0.703 g cm−3
Answers: 3


Physics, 22.06.2019 22:00
The pilot of a helicopter hovers at an altitude of 1200 feet over a park. the angle of depression to the base of a statue is 17 degrees. the angle of depression to the nearest park exit, in line with the statue, is 14 degrees. to the nearest foot, what is the distance from the statue to the exit?
Answers: 1
You know the right answer?
Questions


Computers and Technology, 09.12.2021 03:40




Social Studies, 09.12.2021 03:40

Business, 09.12.2021 03:40


Mathematics, 09.12.2021 03:40

Spanish, 09.12.2021 03:40

Biology, 09.12.2021 03:40

Spanish, 09.12.2021 03:40

Mathematics, 09.12.2021 03:40


Mathematics, 09.12.2021 03:40

Computers and Technology, 09.12.2021 03:40

Mathematics, 09.12.2021 03:40



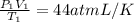
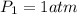
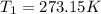
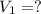
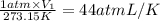
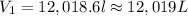
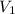 is 12.019 Liters.
is 12.019 Liters.

