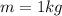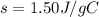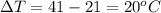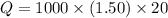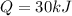Physics, 27.01.2020 19:31 khalid7746
Asample of octane (c8h18) that has a mass of 0.750 g is burned in a bomb calorimeter. as a result, the temperature of the calorimeter increases from 21.0°c to 41.0°c. the specific heat of the calorimeter is 1.50 j/(g • °c), and its mass is 1.00 kg. how much heat is released during the combustion of this sample? use mc021-1.jpg. 22.5 kj 30.0 kj 31.5 kj 61.5 kj

Answers: 2


Another question on Physics

Physics, 22.06.2019 09:00
The pressure proportional to the area a- inversely b- directly c- increase d-decrease
Answers: 2

Physics, 22.06.2019 10:00
(a) calculate the number of electrons in a small, electrically neutral silver pin that has a mass of 10.0 g. silver has 47 electrons per atom, and its molar mass is 107.87 g/mol. (b) imagine adding electrons to the pin until the negative charge has the very large value 1.00 mc. how many electrons are added for every 109 electrons already present
Answers: 3

Physics, 22.06.2019 10:00
Aria drove to the store, did some shopping, and then came home. during maria's trip, when was her displacement equal to zero?
Answers: 1

Physics, 22.06.2019 21:00
What is the efficiency of an engine that does 80 j of work and exhausts 320 j of heat while taking in 400 j of heat ? a. 10% b. 20% c. 80% d. 25%
Answers: 1
You know the right answer?
Asample of octane (c8h18) that has a mass of 0.750 g is burned in a bomb calorimeter. as a result, t...
Questions


English, 14.01.2021 18:00



History, 14.01.2021 18:00

History, 14.01.2021 18:00


Mathematics, 14.01.2021 18:00

Mathematics, 14.01.2021 18:00


Mathematics, 14.01.2021 18:00

Mathematics, 14.01.2021 18:00

Biology, 14.01.2021 18:00

Biology, 14.01.2021 18:00


Mathematics, 14.01.2021 18:00


English, 14.01.2021 18:00

Business, 14.01.2021 18:00