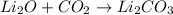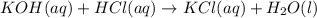Answers: 2


Another question on Physics

Physics, 22.06.2019 04:30
Which of the following is not a characteristic of s waves? a. travel slower than p waves. b. cannot be detected in locations more than 105° from an earthquake’s epicenter. c. travel through solids and liquids. d. only affect coastal regions.
Answers: 2

Physics, 22.06.2019 07:20
If 2 moles of co_2 at 2l and 500k are expanded reversibly to 20l, more work can be obtained from an adiabatic process than from an isothermal process. is the above statement true or false?
Answers: 3

Physics, 22.06.2019 16:30
In a hydrogen molecule there are a total of four charges, 2 protons in the two nuclei, and 2 electrons. how many unique charge-pairs are there (without double counting)?
Answers: 3

You know the right answer?
Potassium hydroxide (koh) and hydrochloric acid (hcl) react in a beaker. they form potassium chlorid...
Questions




Computers and Technology, 21.01.2020 21:31




Computers and Technology, 21.01.2020 21:31









Computers and Technology, 21.01.2020 21:31

Computers and Technology, 21.01.2020 21:31