
Physics, 21.04.2021 23:40 love123jones
Sample of pure boron contains only isotope X and isotope Y.
A nucleus of X has more mass than a nucleus of Y.
[o[4].[4] The sample is ionised, producing ions each with a charge of +1.6 x 10°C.
The specific charge of an ion of X is 8.7 x 10°C kg".
Calculate the mass of an ton of X.
[1 mark]
h
mass of ion = kg
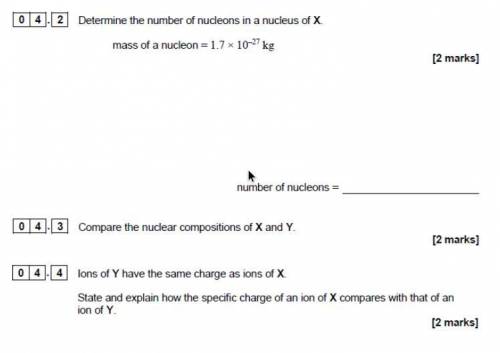
[4].[2] Determine the number of nucleons in a nucleus of X.
mass of a nucleon = 1.7 x 1077 kg
[2 marks]
h
number of nucleons =
[o[4].[3] Compare the nuclear compositions of X and Y.
[2 marks]
[o[4].[4] lons of Y have the same charge as ions of X.
State and explain how the specific charge of an ion of X compares with that of an
ion of Y.
[2 marks]


Answers: 2


Another question on Physics

Physics, 22.06.2019 04:00
What is the final velocity of the initial velocity of 25 and -50?
Answers: 2

Physics, 22.06.2019 05:00
At time t=0, a particle is located at the point (3,6,9). it travels in a straight line to the point (5,2,7), has speed 8 at (3,6,9) and constant acceleration 2i−4j−2k. find an equation for the position vector of the particle.
Answers: 2

Physics, 22.06.2019 05:30
An object weighs 40n in air, weighs 20n when submerged in water and 30n when submerged in a liquid of unknown density. what is the density of the liquid?
Answers: 2

Physics, 22.06.2019 18:00
Which is the most accurate name for the ionic compound cas?
Answers: 1
You know the right answer?
Sample of pure boron contains only isotope X and isotope Y.
A nucleus of X has more mass than a nu...
Questions

Computers and Technology, 29.01.2022 14:00



Business, 29.01.2022 14:00



Mathematics, 29.01.2022 14:00

Mathematics, 29.01.2022 14:00

Mathematics, 29.01.2022 14:00


Computers and Technology, 29.01.2022 14:00



Mathematics, 29.01.2022 14:00


Mathematics, 29.01.2022 14:00


Spanish, 29.01.2022 14:00

English, 29.01.2022 14:00




