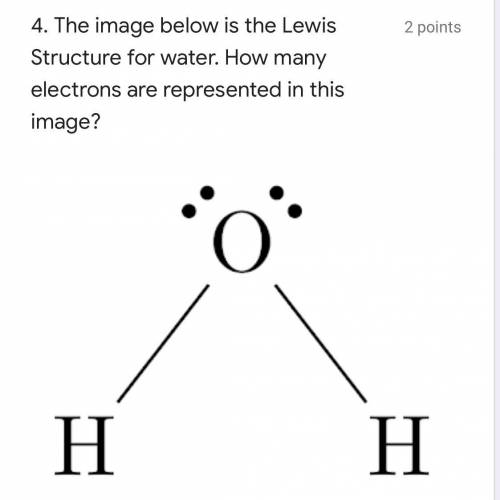
I just need some help with this.
...

Answers: 3


Another question on Physics

Physics, 22.06.2019 15:50
An object with initial temperature 130 ∘ f is submerged in large tank of water whose temperature is 50 ∘ f . find a formula for f ( t ) , the temperature of the object after t minutes, if the cooling constant is k = − 0.2 . remember newton's law of cooling (the rate of change of temperature with respect to time is equal to k times the difference between the temperature of the object and the surrounding temperature) ! : )
Answers: 1

Physics, 22.06.2019 16:20
Iupil u5l aluve time remaining 01: 53: 45 ei and both agreed that childhood experiences play an important role in the development of oneself a sigmund freud alfred adler b. carl jung alfred adler c.carl jung erik erikson d. sigmund freud.. carl jung select the best answer from the choices provided save and exit next submit mark this and return
Answers: 1

Physics, 22.06.2019 18:50
An insulated thermos contains 148 g of water at 72.7 ˚c. you put in a 11.7 g ice cube at 0.00 ˚c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg•k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?
Answers: 2

Physics, 22.06.2019 19:30
A47.2 g block of copper whose temperature is 480 k is placed in an insulating box with a 91.8 g block of lead whose temperature is 200 k. (a) what is the equilibrium temperature of the two-block system? (b) what is the change in the internal energy of the two-block system between the initial state and the equilibrium state? (c) what is the change in the entropy of the two-block system? the heat capacities of copper and lead are 386 j/kg·k and 128 j/kg·k, respectively.
Answers: 1
You know the right answer?
Questions

Mathematics, 21.09.2019 22:50

Mathematics, 21.09.2019 22:50

History, 21.09.2019 22:50





Mathematics, 21.09.2019 22:50


Computers and Technology, 21.09.2019 22:50




Mathematics, 21.09.2019 23:00



English, 21.09.2019 23:00



History, 21.09.2019 23:00