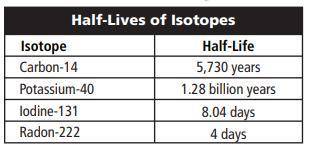
Calculate how much of an 80 g sample of
carbon-14 will be left after 17,190 years.
...


Answers: 1


Another question on Physics

Physics, 22.06.2019 03:00
An internally reversible refrigerator has a modified coefficient of performance accounting for realistic heat transfer processes of where qin is the refrigerator cooling rate, qout is the heat rejection rate, and is the power input. show that copm can be expressed in terms of the reservoir temperatures tc and th, the cold and hot thermal resistances rt,c and rt,h, and qin, as where rtot rt,c rt,h. also, show that the power input may be expressed as 1.39 a household refrigerator operates with cold- and hot-temperature reservoirs of tc 5 c and th 25 c, respectively. when new, the cold and hot side resistances are rc,n 0.05 k/w and rh,n 0.04 k/w, respectively. over time, dust accumulates on the refrigerator’s condenser coil, which is located behind the refrigerator, increasing the hot side resistance to rh,d 0.1 k/w. it is desired to have a refrigerator cooling rate of qin 750 w. using the results of problem 1.38, determine the modified coefficient of performance and the required power input w under (a) clean and (b) dusty coil conditions. internally reversible refrigerator qout qin w high-temperature reservoir low-temperature reservoir th th,i tc,i tc high-temperature side resistance low-temperature side resistance w qin th tc qinrtot tc qinrtot copm tc qinrtot th tc
Answers: 2

Physics, 22.06.2019 16:40
Which are true about beta reactions? there will be more than one answer. a. it has no change b. it doesn't change the mass of the number c. it has a mass of 0.0005 atoms d. it causes transmutation.
Answers: 3

Physics, 22.06.2019 18:50
8.29 two streams containing pyridine and acetic acid at 25°c are mixed and fed into a heat exchanger. due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°c using a stream of chilled ethylene glycol as indicated in the diagram. calculate the mass flow rate of ethylene glycol needed. the heat capacity of ethylene glycol at these conditions is approximately 2.8 kj/(kg k), and the enthalpy change of mixing (δmixh) is given below.
Answers: 3

Physics, 22.06.2019 22:30
Which of these activities increases the amount of carbon in the atmosphere?
Answers: 1
You know the right answer?
Questions

Chemistry, 18.03.2020 02:01

Mathematics, 18.03.2020 02:01


Chemistry, 18.03.2020 02:01



English, 18.03.2020 02:02


Mathematics, 18.03.2020 02:02




Mathematics, 18.03.2020 02:02


Biology, 18.03.2020 02:02

Mathematics, 18.03.2020 02:02

Mathematics, 18.03.2020 02:02



Arts, 18.03.2020 02:03