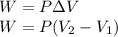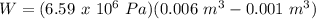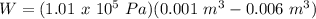A sample of gas, initially with a volume of 1.0 L, undergoes a thermodynamic cycle. Find the work done by the gas on its environment during each stage of the cycle described below. (Enter your answers in J.)
(a) First, the gas expands from a volume of 1.0 L to 6.0 L at a constant pressure of 6.5 atm.
(b) Second, the gas is cooled at constant volume until the pressure falls to 1.0 atm.
(c) Third, the gas is compressed at a constant pressure of 1.0 atm from a volume of 6.0 L to 1.0 L. (Note: Be careful of signs.)
(d) Finally, the gas is heated until its pressure increases from 1.0 atm to 6.5 atm at a constant volume. (e) What is the net work done by the gas on its environment during the complete cycle described above?

Answers: 1


Another question on Physics


Physics, 21.06.2019 21:00
Amarble, a bowling ball, a basketball, and a baseball are rolling across the floor at 10 m/s. which one has the greatest kinetic energy? the marble the bowling ball the basketball the baseball
Answers: 1

Physics, 22.06.2019 18:30
Jenny wants to test the electrical conductivity of two substances dissolved in water. she is preparing the containers for the experiment. which factor is most important for her to control? a) volumes of the solutions b) appearance of the solutions c) temperatures of the solutions d) concentrations of the solutions
Answers: 1

Physics, 22.06.2019 19:30
Juliette sets the initial velocity to +10 m/s, the acceleration to zero, and clicks “start.” how can shakina describe the subsequent motion of the car juliette controls?
Answers: 3
You know the right answer?
A sample of gas, initially with a volume of 1.0 L, undergoes a thermodynamic cycle. Find the work do...
Questions

Mathematics, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01


Mathematics, 20.09.2020 04:01




Mathematics, 20.09.2020 04:01

English, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01


Computers and Technology, 20.09.2020 04:01







English, 20.09.2020 04:01

Chemistry, 20.09.2020 04:01