

Answers: 1


Another question on Physics

Physics, 22.06.2019 09:00
Abicycle slows down when the rider applies the brakes. what type of energy transformation is involved in this example? a. kinetic energy into heat energy b. heat energy into potential energy c. potential energy into kinetic energy d. kinetic energy into mechanical energy
Answers: 1

Physics, 23.06.2019 02:00
Did anyone do 1.06 lab: cloud formation i need with it i go to k12 lavcame i need to get it done quick
Answers: 2

Physics, 23.06.2019 03:00
Agust of wind pushes a beach ball with a force of 9 newtons.the ball rolls 10 meters in 3 seconds. what is the power of the wind?
Answers: 1

Physics, 23.06.2019 10:40
2.which choice most accurately explains what happens to the bonds between atoms when water changes to steam, and what becomes of the energy added during this phase change? when water changes to steam bonds between molecules are created. after reaching the boiling point, energy is used to change bond strength and change the kinetic energy of the atoms. when water changes to steam bonds between molecules break apart. after reaching the boiling point, energy is used to break bonds, but does not affect the kinetic energy of the atoms. when water changes to steam bonds between molecules are created. after reaching the boiling point, energy is used to break bonds, but does not affect the kinetic energy of the atoms. when water changes to steam bonds between molecules break apart. after reaching the boiling point, energy is used to change bond strength and change the kinetic energy of the atoms.
Answers: 1
You know the right answer?
gayle cooks a roast in her microwave oven. the klystron tube in the oven emits photons whose energy...
Questions



History, 27.02.2021 14:00

Mathematics, 27.02.2021 14:00


Mathematics, 27.02.2021 14:00

Biology, 27.02.2021 14:00

Chemistry, 27.02.2021 14:00


Chemistry, 27.02.2021 14:00

Social Studies, 27.02.2021 14:00




Chemistry, 27.02.2021 14:00

Mathematics, 27.02.2021 14:00

Physics, 27.02.2021 14:00


History, 27.02.2021 14:00

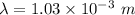
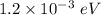 .
.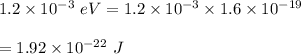
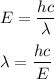
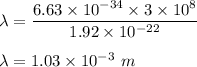
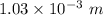 .
.

