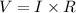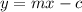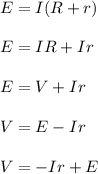A group of students wants to determine the internal resistance of a battery. They connect the battery to a variable resistor. The students measure the potenial differnce across the battery as a function of the current throught the battery as they vary the resistance. Which of the following analyses of the data could be used to determine the internal resistance of the battery?
A. Divide the potential difference across the battery by the current through it for each data point. The average of these calculations gives the internal resistance of the battery.
B. Graph the potential difference across the battery as a function of the current through it. Extrapolate to find the y- intercept and divide this by the average of the current measurements to find the internal resistance of the battery.
C. Find the best-fit straight line for a graph of potential difference across the battery as a function of the current through it. The absolute value of the slope represents the internal resistance of the battery.
D. This data cannot be analyzed to give the internal resistance of the battery, because the potential difference across the battery does not depend on the current.

Answers: 3


Another question on Physics

Physics, 21.06.2019 16:00
In a laboratory, nakisha mixes sodium hydroxide solution with an indicator called phenolphthalein. when combined, they create a pink solution. nakisha wonders if mixing other solutions with phenolphthalein will also create this pink color. how could she use the scientific inquiry process to explore it further?
Answers: 1

Physics, 22.06.2019 00:40
Aballet student who learns with the of his instructor is demonstrating learning.
Answers: 3

Physics, 22.06.2019 03:30
As part of an industrial process, air as an ideal gas at 10 bar, 400k expands at steady state through a valve to a pressure of 4 bar. the mass flow rate of air is 0.5 kg/s. the air then passes through a heat exchanger where it is cooled to a temperature of 295k with negligible change in pressure. the valve can be modeled as a throttling process, and kinetic and potential energy effects can be neglected. (a) for a control volume enclosing the valve and heat exchanger and enough of the local surroundings that the heat transfer occurs at the ambient temperature of 295 k, determine the rate of entropy production, in kw/k. (b) if the expansion valve were replaced by an adiabatic turbine operating isentropically, what would be the entropy production? compare the results of parts (a) and (b) and discuss.
Answers: 3

Physics, 22.06.2019 03:50
Aspecimen of oil having an initial volume of 580cm^3 is subjected to a pressure increase of 4.0 mpa, and the volume is found to decrease by 0.40 cm^3. (a) what is the bulk modulus of the material? (b) what is the compressibility of the material?
Answers: 1
You know the right answer?
A group of students wants to determine the internal resistance of a battery. They connect the batter...
Questions

Chemistry, 21.06.2020 17:57


Chemistry, 21.06.2020 17:57





Mathematics, 21.06.2020 17:57

History, 21.06.2020 17:57



Biology, 21.06.2020 17:57

Mathematics, 21.06.2020 17:57

English, 21.06.2020 17:57

Computers and Technology, 21.06.2020 17:57