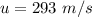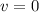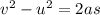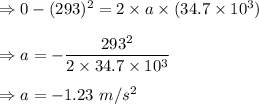Physics, 03.06.2021 03:00 lanashanabJHsbd1099
It is 2058 and you are taking your grandchildren to Mars. At an elevation of 34.7 km above the surface of Mars, your spacecraft is dropping vertically at a speed of 293 m/s. The spacecraft is to make a soft landing -- that is, at the instant it reaches the surface of Mars, its velocity is zero. Assume the spacecraft undergoes constant acceleration from the elevation of 34.7 km until it reaches the surface of Mars. What is the magnitude of the acceleration

Answers: 3


Another question on Physics

Physics, 22.06.2019 13:30
The two stars in a certain binary star system move in circular orbits. the first star, alpha, has an orbital speed of 36 km/s. the second star, beta, has an orbital speed of 12 km/s. the orbital period is 137 d. a) what is the mass of the star alpha? b) what is the mass of the star beta?
Answers: 1

Physics, 22.06.2019 20:50
An ideal otto cycle has a compression ratio of 8. at the beginning of the compression process, air is at 95 kpa and 27°c, and 750 kj/kg of heat is transferred to air during the constant-volume heat-addition process. assuming constant specific heats at room temperature, determine (a) the pressure and temperature at the end of the heat-addition process, (b) the net work output, (c) the thermal efficiency, and (d) the mean effective pressure for the cycle. (4390 kpa, 1730 k; 423 kj/kg; 56.4%; 534 kpa)
Answers: 1

Physics, 23.06.2019 00:50
Aforce of 8,480 n is applied to a cart to accelerate it at a rate of 26.5 m/s2. what is the mass of the cart? 865 kg 320 kg 12.1 kg 3.12 kg
Answers: 1

Physics, 23.06.2019 08:00
Use henry's law and the solubilities given below to calculate the total volume of nitrogen and oxygen gas that should bubble out of 1.7 l of water upon warming from 25 ˚c to 50 ˚c. assume that the water is initially saturated with nitrogen and oxygen gas at 25 ˚c and a total pressure of 1.0 atm. assume that the gas bubbles out at a temperature of 50 ˚c. the solubility of oxygen gas at 50 ˚c is 27.8 mg/l at an oxygen pressure of 1.00 atm. the solubility of nitrogen gas at 50 ˚c is 14.6 mg/l at a nitrogen pressure of 1.00 atm. assume that the air above the water contains an oxygen partial pressure of 0.21 atm and a nitrogen partial pressure of 0.78 atm.
Answers: 2
You know the right answer?
It is 2058 and you are taking your grandchildren to Mars. At an elevation of 34.7 km above the surfa...
Questions

Mathematics, 09.06.2021 07:00

English, 09.06.2021 07:00



Mathematics, 09.06.2021 07:00

Social Studies, 09.06.2021 07:00

Chemistry, 09.06.2021 07:00

History, 09.06.2021 07:00

Mathematics, 09.06.2021 07:00

Mathematics, 09.06.2021 07:00


Mathematics, 09.06.2021 07:00

Mathematics, 09.06.2021 07:00

Physics, 09.06.2021 07:00



Mathematics, 09.06.2021 07:00



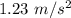
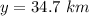 , spacecraft is dropping vertically at a speed of
, spacecraft is dropping vertically at a speed of