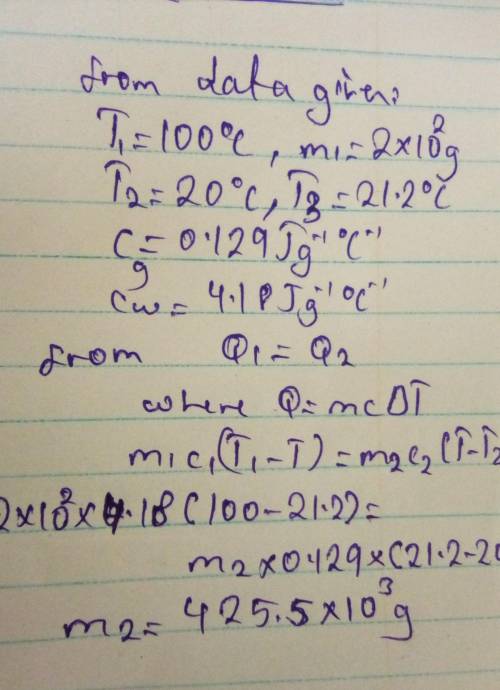Physics, 15.06.2021 14:00 soccerhannah290
Gold at 100.0°C is placed in 2.00×10^2 g of water at 20.0°C. The mixture reaches equilibrium at 21.2°C. the specific heat of gold is 0.129 (J/g°C) What is the mass of the gold? Specific heat of water is 4.18 (J/g°C) .

Answers: 1


Another question on Physics

Physics, 22.06.2019 00:30
Which is not one of the major climate zones? question 3 options: rain forest polar tropical temperate
Answers: 1


Physics, 22.06.2019 21:00
What is the efficiency of an engine that does 80 j of work and exhausts 320 j of heat while taking in 400 j of heat ? a. 10% b. 20% c. 80% d. 25%
Answers: 1

Physics, 23.06.2019 00:30
What is the relationship between wavelength of light and the quantity of energy per photon?
Answers: 2
You know the right answer?
Gold at 100.0°C is placed in 2.00×10^2 g of water at 20.0°C. The mixture reaches equilibrium at 21.2...
Questions


English, 03.12.2020 01:00

Physics, 03.12.2020 01:00


Mathematics, 03.12.2020 01:00




Mathematics, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00





Mathematics, 03.12.2020 01:00

English, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00

English, 03.12.2020 01:00


History, 03.12.2020 01:00