
Physics, 17.06.2021 15:10 montanolumpuy
If the outermost electron in an atom is excited to a very high energy state, its orbit is far beyond that of the other electrons. To a good approximation, we can think of the electron as orbiting a compact core with a charge equal to the charge of a single proton. The outer electron in such a Rydberg atom thus has energy levels corresponding to those of hydrogen.
Sodium is a common element for such studies. How does the radius you calculated in part A compare to the approximately 0.20 nm radius of a typical sodium atom?
r100/rNa = .

Answers: 2


Another question on Physics


Physics, 22.06.2019 15:30
Charge is distributed along the entire x-axis with uniform density λ. how much work does the electric field of this charge distribution do on an electron that moves along the y-axis from y = a to y = b? (use the following as necessary: a, b, ε0, λ, and q for the charge on an electron.)
Answers: 3

Physics, 22.06.2019 21:20
The strong nuclear force acts over a small distance than the electrostatic force. true or false
Answers: 2

Physics, 22.06.2019 22:50
The illuminance of a surface varies inversely with the square of its distance from the light source. if the illuminance of a surface is 120 lumens per square meter when its distance from a certain light source is 6 meters, by how many meters should the distance of the surface from the source be increased to reduce its illuminance to 30 lumens per square meter?
Answers: 3
You know the right answer?
If the outermost electron in an atom is excited to a very high energy state, its orbit is far beyond...
Questions

Computers and Technology, 30.12.2020 07:10



Chemistry, 30.12.2020 07:10



Mathematics, 30.12.2020 07:10

Mathematics, 30.12.2020 07:10

Computers and Technology, 30.12.2020 07:10

English, 30.12.2020 07:10


Biology, 30.12.2020 07:10

Business, 30.12.2020 07:10




Arts, 30.12.2020 07:10

Mathematics, 30.12.2020 07:10


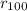 /
/ 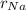 is 2645.0
is 2645.0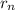 = n²α₀
= n²α₀

