
Two identical cylinders with a movable piston contain 0.7 mol of helium gas at a temperature of 300 K. The temperature of the gas in the first cylinder is increased to 412 K at constant volume by doing work W1 and transferring energy Q1 by heat. The temperature of the gas in the second cylinder is increased to 412 K at constant pressure by doing work W2 while transferring energy Q2 by heat.
Required:
Find ÎEint, 1, Q1, and W1 for the process at constant volume.

Answers: 2


Another question on Physics

Physics, 22.06.2019 19:30
Petroleum contains energy. a. light b. kinetic c. chemical d. mechanical
Answers: 2

Physics, 22.06.2019 20:50
Suppose that a mass of 2 kg is attached a spring whose spring constant is 50. the system is damped such that b = 12 . the mass is set in motion with an initial velocity of -8 m/s at a position 0 meters from equilibrium. set up and solve a differential equation that models this motion. write your solution in the form a cos ( ω t − α ) where α is a positive number. use your solution to fill in the information below: what is the amplitude of the motion? what is the value of ω ? what is the phase shift?
Answers: 1

Physics, 22.06.2019 22:30
Suppose that three astronomical objects, 1, 2, and 3 are observed to lie on a line, and the distance from object 1 to object 3 is d. object 1 has four times the mass of object 3, and seven times the mass of object 2. find the distance between objects 1 and 2 for which the net force on object 2 is zero.
Answers: 1

Physics, 23.06.2019 00:30
Due in 40 explain in terms of frame of reference if you’re inside a moving train everything on the outside can seem to be moving
Answers: 1
You know the right answer?
Two identical cylinders with a movable piston contain 0.7 mol of helium gas at a temperature of 300...
Questions


Business, 23.06.2019 01:30

World Languages, 23.06.2019 01:30



Physics, 23.06.2019 01:30








Biology, 23.06.2019 01:30






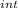 ,₁ = 977.7 J , Q₁ = 977.7 J and W₁ = 0 J
,₁ = 977.7 J , Q₁ = 977.7 J and W₁ = 0 J  = 300 K, T
= 300 K, T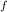 = 412 K, n = 0.7 mol
= 412 K, n = 0.7 mol 

