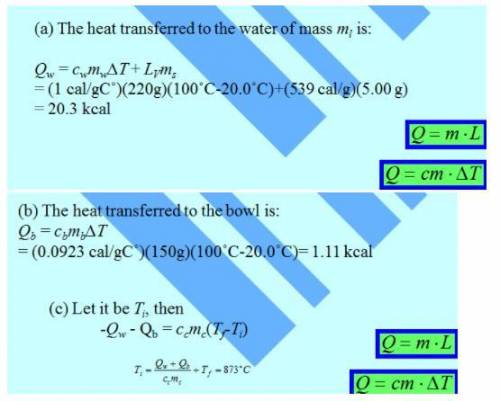
A 150g copper bowl contains 220g of water, both at 20.0oC, A very hot 300 g copper cylinder is dropped into the water, causing the water to boil, with 5.00 g being converted to steam. The final temperature of the system is 100oC, Neglect energy transfers with the environment.
a) How much energy (in calories) is transfered to the water as heat?
b) How much to the bowl?
c) What is the original temperature of the cylinder?

Answers: 1


Another question on Physics

Physics, 21.06.2019 21:30
In what direction does the medium move relative to the direction of the wave? explain.
Answers: 3

Physics, 22.06.2019 18:30
Daughter element the new element produced along with a decay particle in a nuclear transmutation 2. half-life the substance that decays in a nuclear transmutation 3. parent element the change of one chemical element into another by nuclear decay or radioactive bombardment 4. transmutation the time required for the decay of one-half of the atoms in a sample of radioactive material
Answers: 2

Physics, 22.06.2019 18:30
4. now look at the green lines you created by connecting the three boiling point data points and the three melting point data points. for each of these lines, describe any trends you see. 5. locate the elements on your periodic table that you circled in green on your graph. what term or description would you use to identify these elements with respect to the periodic table? 7. using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers.
Answers: 2

Physics, 22.06.2019 19:30
Aspacecraft that renters the earth’s atmosphere drastically slows down. the amount of kinetic energy the spacecraft has as it reenters the earths atmosphere
Answers: 2
You know the right answer?
A 150g copper bowl contains 220g of water, both at 20.0oC, A very hot 300 g copper cylinder is dropp...
Questions

English, 11.07.2019 10:00





History, 11.07.2019 10:00



History, 11.07.2019 10:00



Chemistry, 11.07.2019 10:00

Mathematics, 11.07.2019 10:00

English, 11.07.2019 10:00

Social Studies, 11.07.2019 10:00



Advanced Placement (AP), 11.07.2019 10:00