
Physics, 25.10.2021 01:30 sloansters9315
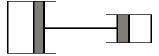
2 pistons are connected like in the picture. I know the area of the big cylinder is 100 cm2cm2 while the one of the small cylinder is 50 cm2 and that the pistons are tied by a rigid wire. There is air in both cylinders, initially having 27°C and normal pressure. The air in the smaller cylinder is heated, it's temperature increasing by 50°C. How much does the temperature of the air in the large cylinder need to increase so that the 2 pistons don't move ?

Answers: 2


Another question on Physics

Physics, 21.06.2019 23:30
A175 g lump of molten lead at its melting point (327 c) is placed into 55.0 g of water at 20.0 c. the specific heat of lead is 130.j/kg c and the hf of lead is 20,400 j/kg. when the lead and the water have reached equilibrium, what is the temperature of the mixture?
Answers: 3

Physics, 22.06.2019 03:00
Which boundary is associated with the building of the himalaya mountains? convergent transform divergent hot spot
Answers: 1

Physics, 22.06.2019 11:10
An isotope undergoes radioactive decay by emitting radiation that has a –1 charge. what other characteristic does the radiation have?
Answers: 3

Physics, 22.06.2019 16:30
The air in an automobile tire with a volume of 2.60 ft3 is at 70°f and 21 psig. determine the amount of air that must be added to raise the pressure to the recommended value of 30 psig. assume the atmospheric pressure to be 14.6 psia and the temperature and the volume to remain constant. the gas constant of air is ru = 53.34ft⋅lbflbm⋅r(1 psia144 lbf/ft2) = 0.3704 psia⋅ft3lbm⋅r the amount of air that must be added to raise the pressure is lbm
Answers: 3
You know the right answer?
2 pistons are connected like in the picture. I know the area of the big cylinder is 100 cm2cm2 while...
Questions



Mathematics, 16.01.2020 19:31

Mathematics, 16.01.2020 19:31

Mathematics, 16.01.2020 19:31


English, 16.01.2020 19:31





Chemistry, 16.01.2020 19:31


Social Studies, 16.01.2020 19:31

Geography, 16.01.2020 19:31


Physics, 16.01.2020 19:31

History, 16.01.2020 19:31


Chemistry, 16.01.2020 19:31



