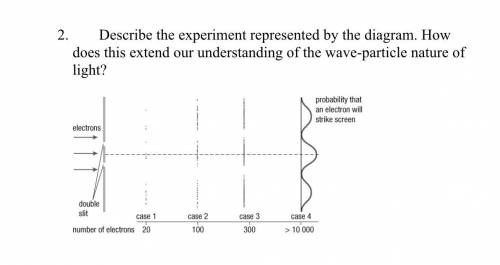
WILL MARK AS THE BRAINLIEST!!!
...

Answers: 3


Another question on Physics

Physics, 22.06.2019 06:30
If an atom contains 11 protons in its nucleus, predict the element with the atomic number and electronic configuration. will the number of electrons remain the same during the chemical reaction in such an atom? explain.
Answers: 3

Physics, 22.06.2019 11:00
1.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the mass defect of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev 2.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the binding energy of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev 3.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the binding energy per nucleon of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev
Answers: 3


Physics, 22.06.2019 13:00
At a certain instant after jumping from the airplane a, a skydiver b is in the position shown and has reached a terminal (constant) speed vb = 52 m/s. the airplane has the same constant speed va = 52 m/s, and after a period of level flight is just beginning to follow the circular path shown of radius ρa = 2330 m. (a) determine the velocity and acceleration of the airplane relative to the skydiver. (b) determine the time rate of change of the speed vr of the airplane and the radius of curvature ρr of its path, both as observed by the nonrotating skydiver.
Answers: 3
You know the right answer?
Questions


English, 17.04.2020 03:49

Mathematics, 17.04.2020 03:49



Biology, 17.04.2020 03:49







Mathematics, 17.04.2020 03:49

Mathematics, 17.04.2020 03:50



Computers and Technology, 17.04.2020 03:50


Mathematics, 17.04.2020 03:50

History, 17.04.2020 03:50