
Physics, 16.01.2022 04:10 nisazaheer
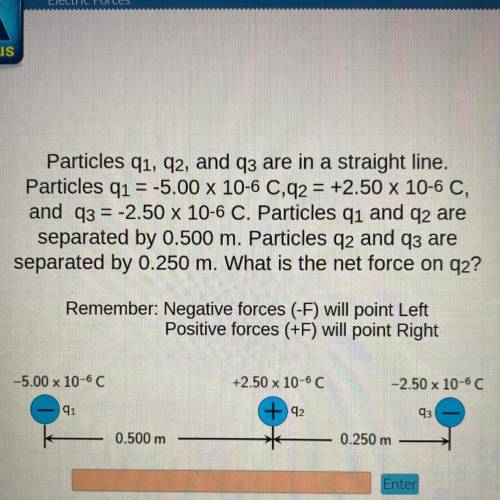
Particles q1, 92, and q3 are in a straight line.
Particles q1 = -5.00 x 10-6 0,92 = +2.50 x 10-6 C,
and q3 = -2.50 x 10-6 C. Particles q1 and q2 are
separated by 0.500 m. Particles q2 and q3 are
separated by 0.250 m. What is the net force on 92?
Remember: Negative forces (-F) will point Left
Positive forces (+F) will point Right
-5.00 x 10-60
-2.50 x 10-6C
+2.50 x 10-6 C
+92
91
93
0.500 m
0.250 m

Answers: 2


Another question on Physics

Physics, 22.06.2019 10:00
Suppose a wheel with a tire mounted on it is rotating at the constant rate of 2.15 times a second. a tack is stuck in the tire at a distance of 0.373 m from the rotation axis. noting that for every rotation the tack travels one circumference (a) find the tack's tangential speed. (b) what is the tacks radial acceleration?
Answers: 2

Physics, 22.06.2019 16:00
Fill in the blanks with the numbers is a measure of the resistance to flow. a high liquid has a high resistance to flow and flows slowly. the ancients thought everything in the world was made of 4 we now know that there are 94 naturally occurring and scientists have created another 24 i am certain they will create even more. honey flows slowly because it has a high to flowing. a can be separated by physical means because it contains more than one pure substance and 2 pure substances are not chemically bonded to each other. a cannot be separated by physical means. all matter is made up of all elements are with the same number of protons. if it is just a single or many bonded together, if all of them have the same number of protons, it is an element. in a piece of pure iron metal, all the are joined together, that piece of iron metal is called elemental iron. a single of iron is called elemental iron. a mixture has differences from place to place. we might need a microscope to see them or they might be obvious to the unaided eye. there are surfaces separating it into different phases. a mixture is the same everywhere. it is uniform. there are no surfaces separating it into different phases. if different kinds of atoms (different elements) are bonded together by their electrons, it is called a there are physical means of to isolate the different pure substances in a mixture and there are chemical means of to isolate the different elements in a compound. 1. element 2. compound 3. mixture 4. heterogeneous 5. homogeneous 6. pure substance 7. atoms 8. separation 9. viscosity 10. resistance
Answers: 3

Physics, 22.06.2019 18:50
8.29 two streams containing pyridine and acetic acid at 25°c are mixed and fed into a heat exchanger. due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°c using a stream of chilled ethylene glycol as indicated in the diagram. calculate the mass flow rate of ethylene glycol needed. the heat capacity of ethylene glycol at these conditions is approximately 2.8 kj/(kg k), and the enthalpy change of mixing (δmixh) is given below.
Answers: 3

Physics, 22.06.2019 20:30
This is a form of winter precipitation. it is frozen precipitation falling as ice pellets. snowflakes melt into raindrops as they pass through a thin layer of warmer air. the raindrops then refreeze into particles of ice when they fall into a layer of sub-freezing air near the surface of the earth. this precipitation is called a) hail. b) rain. c) sleet. d) snow.
Answers: 1
You know the right answer?
Particles q1, 92, and q3 are in a straight line.
Particles q1 = -5.00 x 10-6 0,92 = +2.50 x 10-6 C...
Questions


Mathematics, 12.12.2019 02:31

Mathematics, 12.12.2019 02:31

Mathematics, 12.12.2019 02:31

Mathematics, 12.12.2019 02:31





English, 12.12.2019 02:31


Chemistry, 12.12.2019 02:31

Mathematics, 12.12.2019 02:31

Mathematics, 12.12.2019 02:31


Mathematics, 12.12.2019 02:31


Mathematics, 12.12.2019 02:31

English, 12.12.2019 02:31

Social Studies, 12.12.2019 02:31



