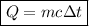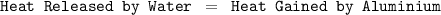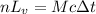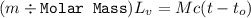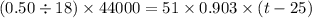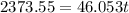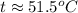Suppose that 0.50 g of water at 25 ∘c condenses on the surface of a 51-g block of aluminum that is initially at 25 ∘c. if the heat released during condensation goes only toward heating the metal, what is the final temperature (in ∘c) of the metal block? (the specific heat capacity of aluminum is 0.903 j/g∘c and the heat of vaporization of water at 25 ∘c is 44.0 kj/mol.)

Answers: 1


Another question on Physics

Physics, 22.06.2019 07:30
Quantum mechanics applies to subatomic, atomic, nanometer-size, and micrometer-size systems. nanometer, micrometer, and kilometer-size systems. atomic, nanometer-size, and micrometer-size systems. subatomic, atomic, and nanometer-size systems.
Answers: 2

Physics, 23.06.2019 01:00
What is the role of the water cycle in maintaining freshwater levels in lake and rivers?
Answers: 1

Physics, 23.06.2019 02:00
Which statement describes the formation of our sun? a disk formed of long trails of stars coiled into a spiral. it formed an elliptical flattened disk. heat and gases contracted within a nebula. gravity pushed stars away from a center core.
Answers: 1

Physics, 23.06.2019 11:30
In a house all the electrical wires run back to either a bank or a blank
Answers: 1
You know the right answer?
Suppose that 0.50 g of water at 25 ∘c condenses on the surface of a 51-g block of aluminum that is i...
Questions

History, 11.01.2022 14:00

Mathematics, 11.01.2022 14:00

Mathematics, 11.01.2022 14:00

Chemistry, 11.01.2022 14:00

Mathematics, 11.01.2022 14:00

History, 11.01.2022 14:00


Mathematics, 11.01.2022 14:00

Computers and Technology, 11.01.2022 14:00


English, 11.01.2022 14:00

Physics, 11.01.2022 14:00

Mathematics, 11.01.2022 14:00

Mathematics, 11.01.2022 14:00


Mathematics, 11.01.2022 14:00

Mathematics, 11.01.2022 14:00