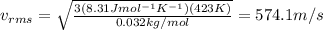Physics, 26.07.2019 03:30 trinity7265
The average kinetic energy of the molecules in a gas sample depends only on the temperature, t. but given the same kinetic energies, a lighter molecule will move faster than a heavier molecule. what is the rms speed of o2 molecules at 423 k?

Answers: 1


Another question on Physics

Physics, 22.06.2019 03:30
Starting with only the balmer series light (visible light), how could we ensure that the solar panels generate a current that mark can use for his power station? a)by gradually increasing the brightness (amount) of light that we shine on it. b)by gradually increasing the frequency of the light we shine on it. c)by gradually increasing the wavelength of the light that we shine on it.
Answers: 3


Physics, 22.06.2019 08:00
If a balloon is taken outside on a very cold day, what will occur?
Answers: 1

Physics, 22.06.2019 21:20
An electron is ejected into a horizontal uniform e⃗ field at a parallel horizontal velocity of v0. assume the electron's initial position x0, initial velocity v0, time t, magnitude of electric field e, electron's mass m, and the magnitude of the electron's charge |e|. ignore the force that earth exerts on the electron. assume the e⃗ field is in the same direction as the initial velocity. part a define the equation for the electron's velocity. express your answer in terms of the variables v0, |e|, t, e, and m.
Answers: 3
You know the right answer?
The average kinetic energy of the molecules in a gas sample depends only on the temperature, t. but...
Questions

Biology, 07.10.2021 19:20



Law, 07.10.2021 19:20


Biology, 07.10.2021 19:20

Mathematics, 07.10.2021 19:20

Mathematics, 07.10.2021 19:20

Advanced Placement (AP), 07.10.2021 19:20


History, 07.10.2021 19:30

Advanced Placement (AP), 07.10.2021 19:30

Mathematics, 07.10.2021 19:30


Social Studies, 07.10.2021 19:30





Engineering, 07.10.2021 19:30

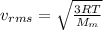
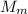 is the molar mass of the gas.
is the molar mass of the gas.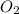 , the molar mass is
, the molar mass is 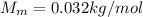 , so since the temperature of the gas in the problem is T=423 K, the rms speed of its molecules is
, so since the temperature of the gas in the problem is T=423 K, the rms speed of its molecules is