
Physics, 25.07.2019 11:30 babyjulie61
The mass of an electron is 9.11×10−31 kg. if the de broglie wavelength for an electron in a hydrogen atom is 3.31×10−10 m, how fast is the electron moving relative to the speed of light? the speed of light is 3.00×108 m/s. express your answer numerically as a percent. view available hint(s)

Answers: 1


Another question on Physics

Physics, 21.06.2019 17:00
The density of a substance is 4.0 g/cm3. if a sample of the substance has a volume of 25 cm3 then what is the mass
Answers: 1

Physics, 21.06.2019 22:30
Under conditions for which the same room temperature is maintained by a heating or cooling system, it is not uncommon for a person to feel chilled in the winter but comfortable in the summer. provide a plausible explanation for this situation (with supporting calculations) by considering a room whose air temperature is maintained at 20â°c throughout the year, while the walls of the room are nominally at 27â°c and 14â°c in the summer and winter, respectively. the exposed surface of a person in the room may be assumed to be at a temperature of 32â°c throughout the year and to have an emissivity of 0.90. the coefficient associated with heat transfer by natural convection between the person and the room air is approximately 2 w/m2 â‹…â‹… k. what is the ratio of the thermal resistance due to convection to the thermal resistance due to radiation in the summer? what is the ratio of thermal resistances in the winter
Answers: 1


Physics, 22.06.2019 09:30
The necleus of an atom is made up of what subatomic particles?
Answers: 1
You know the right answer?
The mass of an electron is 9.11×10−31 kg. if the de broglie wavelength for an electron in a hydrogen...
Questions

Social Studies, 10.10.2019 12:50

English, 10.10.2019 12:50

English, 10.10.2019 12:50

Physics, 10.10.2019 12:50

Mathematics, 10.10.2019 12:50



Mathematics, 10.10.2019 12:50

Biology, 10.10.2019 12:50

English, 10.10.2019 12:50

English, 10.10.2019 12:50




Health, 10.10.2019 12:50

Biology, 10.10.2019 12:50



Social Studies, 10.10.2019 12:50

Mathematics, 10.10.2019 12:50




 m/s
m/s % =
% = 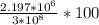 % = 0.732%
% = 0.732%

