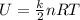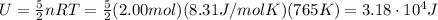Physics, 23.07.2019 21:00 ariana0517
What is the internal energy of 2.00 mol of an ideal diatomic gas at 765 k , assuming all degrees of freedom are active?

Answers: 1


Another question on Physics

Physics, 21.06.2019 22:40
The desk has a weight of 75 lb and a center of gravity at g. determine the initial acceleration of a desk when the man applies enough force f to overcome the static friction at a and b. also, find the vertical reactions on each of the two legs at a and at b. the coefficients of static and kinetic friction at a and b are ms = 0.5 and mk = 0.2, respectively
Answers: 2

Physics, 22.06.2019 01:20
An engineer designs a roller coaster so that a car travels horizontally for 152 ft, then climbs 137 ft at an angle of 34.0° above the horizontal. it then moves 137 ft at an angle of 48.0° below the horizontal. if we take the initial horizontal motion of the car to be along the +x-axis, what is the car's displacement? (give the magnitude of your answer, in ft, to at least four significant figures and give the direction of your answer in degrees counterclockwise from the +x-axis.)
Answers: 1


Physics, 22.06.2019 11:00
1.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the mass defect of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev 2.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the binding energy of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev 3.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the binding energy per nucleon of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev
Answers: 3
You know the right answer?
What is the internal energy of 2.00 mol of an ideal diatomic gas at 765 k , assuming all degrees of...
Questions

Mathematics, 05.08.2021 23:30


Mathematics, 05.08.2021 23:30









French, 05.08.2021 23:30

Mathematics, 05.08.2021 23:30



Mathematics, 05.08.2021 23:30

Mathematics, 05.08.2021 23:30