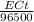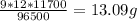Answers: 1


Another question on Physics

Physics, 22.06.2019 00:40
Electroplating is a way to coat a complex metal object with a very thin (and hence inexpensive) layer of a precious metal, such as silver or gold. in essence the metal object is made the cathode of an electrolytic cell in which the precious metal cations are dissolved in aqueous solution. suppose a current of 480.ma is passed through an electroplating cell with an aqueous solution of agno3 in the cathode compartment for 46.0 seconds. calculate the mass of pure silver deposited on a metal object made into the cathode of the cell. round your answer to 3 significant digits. also, be sure your answer contains a unit symbol. ×10μ
Answers: 3

Physics, 22.06.2019 05:00
Aperson stands on a platform, initially at rest, that can rotate freely without friction. the moment of inertia of the person plus the platform is ip. the person holds a spinning bicycle wheel with its axis horizontal. the wheel has moment of inertia iw and angular velocity ωw. take the ωw direction counterclockwise when viewed from above. part a what will be the angular velocity ωp of the platform if the person moves the axis of the wheel so that it points vertically upward?
Answers: 1

Physics, 22.06.2019 05:30
Explain how the energy of rubber ball is tranforned as it roll down a ramp. give evidence that the energy of the og the ball remains the same at all points on the ramp
Answers: 2

Physics, 22.06.2019 14:30
Aracecar driver has to hold on tightly when going around a banked curve. approximately what is the centripetal force on a 2220.0 kg car going around a circle with a diameter of 190.0 meters at 25.0 m/s?
Answers: 1
You know the right answer?
The electrolysis of molten alcl3 for 3.25 hr with an electrical current of 12.0 a produces g of alu...
Questions


Chemistry, 11.10.2019 02:00


English, 11.10.2019 02:00



Chemistry, 11.10.2019 02:00

English, 11.10.2019 02:00


Biology, 11.10.2019 02:00

Mathematics, 11.10.2019 02:00


Mathematics, 11.10.2019 02:00

English, 11.10.2019 02:00



History, 11.10.2019 02:00

Biology, 11.10.2019 02:00


Health, 11.10.2019 02:00