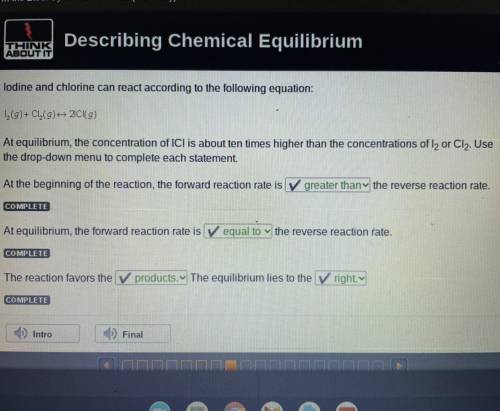
Lodine and chlorine can react according to the following equation:
I2(g) + Cl2(g) 2ICI (g)
A...

Biology, 20.04.2021 18:10 supermkimbrel
Lodine and chlorine can react according to the following equation:
I2(g) + Cl2(g) 2ICI (g)
At equilibrium, the concentration of ICl is about ten times higher than the concentrations of I2 or Cl2. Use
the drop-down menu to complete each statement.
At the beginning of the reaction, the forward reaction rate is
greater thany the reverse reaction rate.

Answers: 2


Another question on Biology

Biology, 22.06.2019 07:30
Nh3 +02-no + h20 is unbalanced what is the balanced equation
Answers: 2

Biology, 22.06.2019 08:30
Pink fur (p) is dominant to purple fur (p) in hamsters. two heterozygous pink hamsters are crossed. what is the probability that these two parents will have an offspring with purple fur? 2/4, 1/2 or 50% 1/4 or 25% 3/4 or 75% 4/4 or 100% there is no chance for this type of offspring
Answers: 1

Biology, 22.06.2019 11:00
Consider the venn diagram of plant reproduction. where in this image, areas a - d, would you insert the picture of the orange lily?
Answers: 1

Biology, 22.06.2019 11:30
Discuss cell surface transport in the structure of constituents
Answers: 1
You know the right answer?
Questions


















Computers and Technology, 14.04.2020 23:06


English, 14.04.2020 23:06



