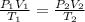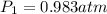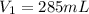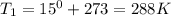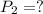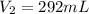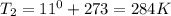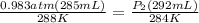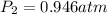Chemistry, 14.07.2019 02:30 ethanyayger
Astudent collects 285 ml of o2 gas at a temperature of 15°c and a pressure of 0.983 atm. the next day, the same sample occupies 292 ml at a temperature of 11°c. what is the new pressure of the gas? 0.946 atm 1.00 atm 0.704 atm

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
Astudent collects 285 ml of o2 gas at a temperature of 15°c and a pressure of 0.983 atm. the next da...
Questions


Mathematics, 18.12.2020 01:10

Mathematics, 18.12.2020 01:10


History, 18.12.2020 01:10




Medicine, 18.12.2020 01:10

Social Studies, 18.12.2020 01:10


History, 18.12.2020 01:10

Mathematics, 18.12.2020 01:10

Health, 18.12.2020 01:10

Mathematics, 18.12.2020 01:10




Health, 18.12.2020 01:10