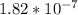Chemistry, 13.07.2019 21:30 ivanyeli4520
The equilibrium concentrations of the reactants and products are [ha] = 0.220 m [h3o ] = 2.00 × 10–4 m [a–] = 2.00 × 10–4 m calculate the ka value for the acid ha.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3
You know the right answer?
The equilibrium concentrations of the reactants and products are [ha] = 0.220 m [h3o ] = 2.00 × 10–4...
Questions










Computers and Technology, 27.11.2019 00:31










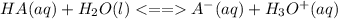
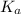 is the equilibrium constant for this equation, which is referred to as the acid dissociation constant.
is the equilibrium constant for this equation, which is referred to as the acid dissociation constant. ![K_{a} = \frac{[H_{3}O^{+}][A^{-}]}{[HA]}](/tpl/images/0086/2201/14aa4.png)