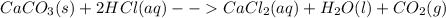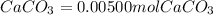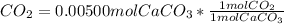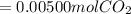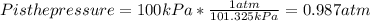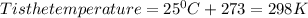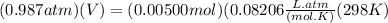Chemistry, 13.07.2019 18:30 auzriannamarie
Chris the chemist works in a laboratory in which the temperature is maintained at a constant 25oc and the pressure is always 100 kpa. chris needs to analyse some calcium carbonate, caco3(s), to determine whether it is pure or has been contaminated. chris will analyse the calcium carbonate by taking a small 0.00500 mole sample and adding hydrochloric acid, hcl(aq), to it until all the calcium carbonate has disappeared and no more carbon dioxide gas, co2(g), is produced. as the gas is produced it will be collected by a water displacement method. the balanced chemical equation for this reaction is known to be: caco3(s) + 2hcl(aq) → cacl2(aq) + co2(g) + h2o(l) if the sample is pure, what volume of carbon dioxide gas will be collected?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
Chris the chemist works in a laboratory in which the temperature is maintained at a constant 25oc an...
Questions


Mathematics, 12.12.2020 16:50

Arts, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50

Computers and Technology, 12.12.2020 16:50

English, 12.12.2020 16:50

History, 12.12.2020 16:50




Advanced Placement (AP), 12.12.2020 16:50

Arts, 12.12.2020 16:50



Mathematics, 12.12.2020 16:50