
Let us assume that fe(oh)2(s) is completely insoluble, which signifies that the precipitation reaction with naoh(aq) (presented in the transition) would go to completion. fe2+(aq)+2naoh(aq) → fe(oh)2(s)+2na+(aq) if you had a 0.500 l solution containing 0.0230 m of fe2+(aq), and you wished to add enough 1.29 m naoh(aq) to precipitate all of the metal, what is the minimum amount of the naoh(aq) solution you would need to add? assume that the naoh(aq) solution is the only source of oh−(aq) for the precipitation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

You know the right answer?
Let us assume that fe(oh)2(s) is completely insoluble, which signifies that the precipitation reacti...
Questions




Mathematics, 21.07.2020 14:01





Mathematics, 21.07.2020 14:01






Mathematics, 21.07.2020 14:01

Chemistry, 21.07.2020 14:01





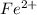 is as follows:-
is as follows:-![Fe^{2+} = 500 mL Fe^{2+} * \frac{0.0230mole\ Fe^{2+}]}{[1 mol\ Fe^{2+}}](/tpl/images/0082/8691/681c2.png) = 11.50 mmol Fe^(2+)
= 11.50 mmol Fe^(2+)![11.50\ mmol\ Fe^{2+} * \frac{[2 \ mol\ NaOH]}{[1 \mol Fe^{2+}}](/tpl/images/0082/8691/9368e.png) = 23.00 mmol NaOH
= 23.00 mmol NaOH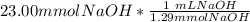 = 17.8 mL NaOH.
= 17.8 mL NaOH.

